PR Newswire09.27.18
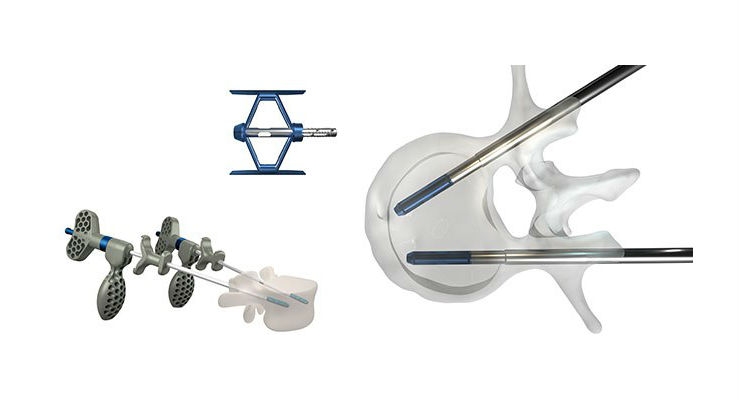
Stryker announced that it has received FDA 510(k) clearance to market the SpineJack Implantable Fracture Reduction System. The SpineJack system is indicated for use in the reduction of painful osteoporotic vertebral compression fractures.
In the SAKOS clinical study, the SpineJack system demonstrated superiority to balloon kyphoplasty (BKP) for the endpoints of freedom from adjacent level fracture and midline vertebral height restoration. The SpineJack system also showed substantial and sustained improvement in both pain (VAS) and function (Oswestry Disability Index) over BKP.
Available in three sizes to accommodate different vertebral body sizes, the SpineJack titanium implant is inserted and expanded, and PMMA bone cement is injected at low pressure to stabilize the restored vertebral body.
The SpineJack system has been commercially available in Europe since 2008 and over 70,000 units have been implanted worldwide. The company plans to execute a limited launch of the device for the remainder of 2018.
For more information, please visit Stryker's booth #1401 at the annual meeting of the North American Spine Society in Los Angeles September 26th - 29th.
In the SAKOS clinical study, the SpineJack system demonstrated superiority to balloon kyphoplasty (BKP) for the endpoints of freedom from adjacent level fracture and midline vertebral height restoration. The SpineJack system also showed substantial and sustained improvement in both pain (VAS) and function (Oswestry Disability Index) over BKP.
Available in three sizes to accommodate different vertebral body sizes, the SpineJack titanium implant is inserted and expanded, and PMMA bone cement is injected at low pressure to stabilize the restored vertebral body.
The SpineJack system has been commercially available in Europe since 2008 and over 70,000 units have been implanted worldwide. The company plans to execute a limited launch of the device for the remainder of 2018.
For more information, please visit Stryker's booth #1401 at the annual meeting of the North American Spine Society in Los Angeles September 26th - 29th.