GlobeNewswire10.19.18
OrthoPediatrics Corp., a company exclusively focused on advancing the field of pediatric orthopedics, announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new RESPONSE 4.5/5.0mm System for treating smaller stature younger patients with complex scoliosis. The system represents the company’s 26th surgical system and is expected to launch in the fourth quarter of 2018.
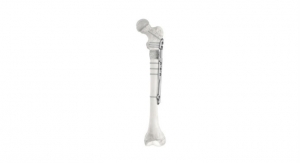
The RESPONSE 4.5/5.0mm System was designed in collaboration with pediatric orthopedic surgeons focused on additional solutions for treating complex pediatric scoliosis patients. It expands the company’s RESPONSE platform to treat smaller stature children and builds upon the successful implant and instrument technology of the RESPONSE 5.5/6.0mm System. The RESPONSE 4.5/5.0mm System offers a hybrid implant technology allowing the option of either a 4.5mm rod in CoCr or 5.0mm rod in titanium or cobalt chromium/chrome material, multiple implant connector options, and innovative, new instrumentation.
OrthoPediatrics’ executive vice president, David Bailey, stated, “We are pleased with the FDA 510(k) clearance for our 4.5/5.0mm system, which allows physicians to better treat smaller statures and patients at a younger age. Our engineering teams have been diligently working with a prominent group of surgeons, and we are excited to bring their innovative vision to life with another surgical solution for treating complex pediatric scoliosis. The addition of the new system to our scoliosis platform continues to demonstrate OrthoPediatrics’ focus and commitment to providing solutions for children with complex spinal disorders.”
Scott Luhmann, M.D., Chief of Staff at Shriners Hospital for Children-St. Louis and Associate Professor in the Department of Orthopedic Surgery and Fellowship Director of Pediatric Orthopedic Surgery at Washington University School of Medicine, commented, “The new Response 4.5/5.0mm System brings OrthoPediatrics’ expertise and passion for musculoskeletal care of pediatric patients to the area of spinal deformity. This surgeon-centric system is focused on surgical efficacy and efficiency with an elegant rod reduction tool that significantly cuts down laborious task time in the effort to provide optimal patient outcome.”
The RESPONSE 4.5/5.0mm System was designed in collaboration with pediatric orthopedic surgeons focused on additional solutions for treating complex pediatric scoliosis patients. It expands the company’s RESPONSE platform to treat smaller stature children and builds upon the successful implant and instrument technology of the RESPONSE 5.5/6.0mm System. The RESPONSE 4.5/5.0mm System offers a hybrid implant technology allowing the option of either a 4.5mm rod in CoCr or 5.0mm rod in titanium or cobalt chromium/chrome material, multiple implant connector options, and innovative, new instrumentation.
OrthoPediatrics’ executive vice president, David Bailey, stated, “We are pleased with the FDA 510(k) clearance for our 4.5/5.0mm system, which allows physicians to better treat smaller statures and patients at a younger age. Our engineering teams have been diligently working with a prominent group of surgeons, and we are excited to bring their innovative vision to life with another surgical solution for treating complex pediatric scoliosis. The addition of the new system to our scoliosis platform continues to demonstrate OrthoPediatrics’ focus and commitment to providing solutions for children with complex spinal disorders.”
Scott Luhmann, M.D., Chief of Staff at Shriners Hospital for Children-St. Louis and Associate Professor in the Department of Orthopedic Surgery and Fellowship Director of Pediatric Orthopedic Surgery at Washington University School of Medicine, commented, “The new Response 4.5/5.0mm System brings OrthoPediatrics’ expertise and passion for musculoskeletal care of pediatric patients to the area of spinal deformity. This surgeon-centric system is focused on surgical efficacy and efficiency with an elegant rod reduction tool that significantly cuts down laborious task time in the effort to provide optimal patient outcome.”