U.S. Department of Justice11.15.19
The United States has filed a complaint against Sioux Falls, S.D., neurosurgeon Wilson Asfora M.D., Medical Designs LLC, and Sicage LLC, alleging False Claims Act violations arising from the alleged payment of kickbacks to Asfora tied to the devices he used in spinal surgeries, the Justice Department announced. Medical Designs and Sicage are medical device distributorships in South Dakota owned and operated by Asfora.
The Anti‑Kickback Statute prohibits offering or paying anything of value to induce the referral of items or services covered by Medicare, Medicaid, and other federal healthcare programs. The government’s complaint alleges that Asfora, Medical Designs, and Sicage engaged in multiple kickback schemes designed to pay Asfora hundreds of thousands of dollars in exchange for Asfora using spinal devices distributed by Medical Designs and Sicage in his spine surgeries. Despite receiving numerous warnings that he was performing medically unnecessary procedures with the devices in which he had a financial interest, Asfora allegedly continued to perform such procedures while personally profiting from his use of devices sold by Medical Designs and Sicage. The United States previously resolved related civil claims against several Sanford Health entities in October 2019.
“The Department of Justice will seek to hold accountable physicians and medical device companies that receive or pay illegal kickbacks in any form,” said Assistant Attorney General Jody Hunt of the Department of Justice’s Civil Division. “Improper inducements have no place in our federal healthcare system where medical decisions should be based on the healthcare needs of patients and not on a physician’s personal financial interest.”
“Our office will aggressively pursue anyone who colludes to violate federal law and compromise the integrity of our healthcare system,” said U.S. Attorney Ron Parsons for the District of South Dakota.
“Government health program patients should be confident that surgical procedures are medically needed, not performed to increase physician profits,” said Curt L. Muller, Special Agent in Charge of the Office of Inspector General at the U.S. Department of Health and Human Services. “For years our fraud alert has warned that physician distributorships are inherently suspect under the Anti-Kickback statute.”
Asfora founded Medical Designs LLC in 1999 after his idea for an existing device modification was rejected by a major medical device corporation, according to the company's website. Medical Designs has developed four products: The Asfora Bullet Cage, the Dakota Knife, the Odontoid Curved Drill Guide, and Asfora Anterior Cervical Plate System.
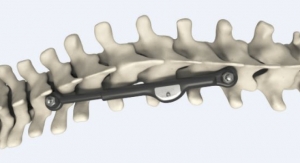
The Asfora Bullet Cage is indicated for spinal fusion procedures in patients with degenerative disc disease (DDD) and instability in the lumbar spine at one or two contiguous levels from L2 to S1. The Dakota Knife Blade is a stainless steel surgical instrument designed for use in open, limited open, and minimally invasive carpal tunnel release surgery. The Dakota Knife Blade is a single-use, disposable cutting instrument for transecting the transverse carpal ligament, product literature on the company's website states.
The Odontoid Curved Drill Guide is a curved, stainless steel tube with reversible handle to accommodate both left-handed and right-handed surgeons, and the Asfora Anterior Cervical Plate System (cleared by the U.S. Food and Drug Administration in 2015) is intended to provide temporary stabilization to the anterior spine during the development of cervical spine fusions (C2-T1) for the following indications: degenerative disc disease (DDD) (defined as neck pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e., fracture or dislocation), spinal stenosis, deformities or curvatures (i.e., scoliosis, kyphosis, and/or lordosis), tumor, pseudoarthrosis, and failed previous fusion.
The SICAGE System, meanwhile is designed to provide a minimally invasive approach to treating sacroiliac joint dysfunction. The product was cleared by the FDA in 2017.
The United States filed its complaint in a lawsuit pending in the U.S. District Court for the District of South Dakota that was filed under the qui tam, or whistleblower, provisions of the False Claims Act. Under the act, a private citizen can sue on behalf of the government and receive a share of any recovery. The act permits the United States to intervene and take over responsibility for litigating the case, as it has done here. Those who violate the act are subject to treble damages and penalties.
This matter is being handled by the Civil Division’s Commercial Litigation Branch and the U.S. Attorney’s Office for the District of South Dakota, with assistance from the Department of Health and Human Service’s Office of Inspector General.
The case is captioned United States ex rel. Bechtold, et al. v. Asfora, et al., No. 4:16-cv-04115-LLP (D.S.D.). The claims asserted against the defendants are allegations only, and there has been no determination of liability.
The Anti‑Kickback Statute prohibits offering or paying anything of value to induce the referral of items or services covered by Medicare, Medicaid, and other federal healthcare programs. The government’s complaint alleges that Asfora, Medical Designs, and Sicage engaged in multiple kickback schemes designed to pay Asfora hundreds of thousands of dollars in exchange for Asfora using spinal devices distributed by Medical Designs and Sicage in his spine surgeries. Despite receiving numerous warnings that he was performing medically unnecessary procedures with the devices in which he had a financial interest, Asfora allegedly continued to perform such procedures while personally profiting from his use of devices sold by Medical Designs and Sicage. The United States previously resolved related civil claims against several Sanford Health entities in October 2019.
“The Department of Justice will seek to hold accountable physicians and medical device companies that receive or pay illegal kickbacks in any form,” said Assistant Attorney General Jody Hunt of the Department of Justice’s Civil Division. “Improper inducements have no place in our federal healthcare system where medical decisions should be based on the healthcare needs of patients and not on a physician’s personal financial interest.”
“Our office will aggressively pursue anyone who colludes to violate federal law and compromise the integrity of our healthcare system,” said U.S. Attorney Ron Parsons for the District of South Dakota.
“Government health program patients should be confident that surgical procedures are medically needed, not performed to increase physician profits,” said Curt L. Muller, Special Agent in Charge of the Office of Inspector General at the U.S. Department of Health and Human Services. “For years our fraud alert has warned that physician distributorships are inherently suspect under the Anti-Kickback statute.”
Asfora founded Medical Designs LLC in 1999 after his idea for an existing device modification was rejected by a major medical device corporation, according to the company's website. Medical Designs has developed four products: The Asfora Bullet Cage, the Dakota Knife, the Odontoid Curved Drill Guide, and Asfora Anterior Cervical Plate System.
The Asfora Bullet Cage is indicated for spinal fusion procedures in patients with degenerative disc disease (DDD) and instability in the lumbar spine at one or two contiguous levels from L2 to S1. The Dakota Knife Blade is a stainless steel surgical instrument designed for use in open, limited open, and minimally invasive carpal tunnel release surgery. The Dakota Knife Blade is a single-use, disposable cutting instrument for transecting the transverse carpal ligament, product literature on the company's website states.
The Odontoid Curved Drill Guide is a curved, stainless steel tube with reversible handle to accommodate both left-handed and right-handed surgeons, and the Asfora Anterior Cervical Plate System (cleared by the U.S. Food and Drug Administration in 2015) is intended to provide temporary stabilization to the anterior spine during the development of cervical spine fusions (C2-T1) for the following indications: degenerative disc disease (DDD) (defined as neck pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e., fracture or dislocation), spinal stenosis, deformities or curvatures (i.e., scoliosis, kyphosis, and/or lordosis), tumor, pseudoarthrosis, and failed previous fusion.
The SICAGE System, meanwhile is designed to provide a minimally invasive approach to treating sacroiliac joint dysfunction. The product was cleared by the FDA in 2017.
The United States filed its complaint in a lawsuit pending in the U.S. District Court for the District of South Dakota that was filed under the qui tam, or whistleblower, provisions of the False Claims Act. Under the act, a private citizen can sue on behalf of the government and receive a share of any recovery. The act permits the United States to intervene and take over responsibility for litigating the case, as it has done here. Those who violate the act are subject to treble damages and penalties.
This matter is being handled by the Civil Division’s Commercial Litigation Branch and the U.S. Attorney’s Office for the District of South Dakota, with assistance from the Department of Health and Human Service’s Office of Inspector General.
The case is captioned United States ex rel. Bechtold, et al. v. Asfora, et al., No. 4:16-cv-04115-LLP (D.S.D.). The claims asserted against the defendants are allegations only, and there has been no determination of liability.