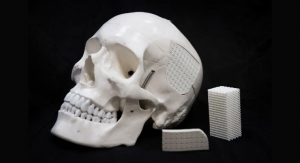
PR Newswire09.23.20
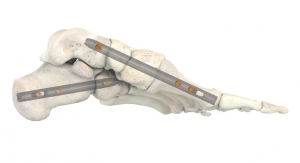
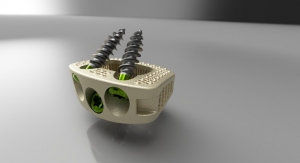
RTI Surgical recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Dynamic Active Compression (DAC) Plate to provide stabilization and fixation of small bones of the foot. The DAC plate addresses an unmet need in the foot and ankle market and represents RTI Surgical's first 510(k) Clearance since the company became an independent player with the support of Montagu.
"The DAC plate is designed to improve fusion procedures in the foot by combining the rigid stability of a plate with Nitinol's ability to provide continuous compression and ensure apposition of the bony surfaces during healing," said Dr. Constantine Demetracopoulos, Hospital for Special Surgery. The system's simplicity also provides for surgical application using minimal, straight-forward steps which address challenges of current standard plate and Nitinol staple options, without the complexity associated with more advanced options.
"The DAC plate is a prime example of how we are able to leverage our core capabilities to enter a new market. Our trauma experience was crucial in creating the first hardware product we specifically designed for lower extremities in the foot and ankle space. We look forward to continuing to bring new technologies to the market to address unmet needs and help patients," said Jimmy Blanchard, Vice President of Sales.
RTI is dedicated to beginning development and design of the novel patent-pending active compression technology for other applications. As an industry-leading surgical implant supplier, RTI partners with leading medical technology companies to design and develop medical devices. RTI also designs, develops and manufactures novel technologies which are commercialized through multiple distribution channels.
"The DAC plate is designed to improve fusion procedures in the foot by combining the rigid stability of a plate with Nitinol's ability to provide continuous compression and ensure apposition of the bony surfaces during healing," said Dr. Constantine Demetracopoulos, Hospital for Special Surgery. The system's simplicity also provides for surgical application using minimal, straight-forward steps which address challenges of current standard plate and Nitinol staple options, without the complexity associated with more advanced options.
"The DAC plate is a prime example of how we are able to leverage our core capabilities to enter a new market. Our trauma experience was crucial in creating the first hardware product we specifically designed for lower extremities in the foot and ankle space. We look forward to continuing to bring new technologies to the market to address unmet needs and help patients," said Jimmy Blanchard, Vice President of Sales.
RTI is dedicated to beginning development and design of the novel patent-pending active compression technology for other applications. As an industry-leading surgical implant supplier, RTI partners with leading medical technology companies to design and develop medical devices. RTI also designs, develops and manufactures novel technologies which are commercialized through multiple distribution channels.