Sam Brusco, Associate Editor10.14.22
Nevro has gained U.S. Food and Drug Administration (FDA) approval for its Senza HFX iQ spinal cord stimulation (SCS) system.
Senza HFX iQ is an artificial intelligence (AI)-based SCS system that learns from patients, developed to address pain variability and help patients optimize and maintain long-term pain relief. The system is composed of the HFX implantable pulse generator (IPG), HFX trial stimulator, charger, and HFX app.
The system will launch with apps tailored for chronic back and leg pain, including non-surgical back pain and painful diabetic neuropathy. Senza HFX iQ was designed to optimize care by collecting data and guiding through a customized treatment pathway. Features include:
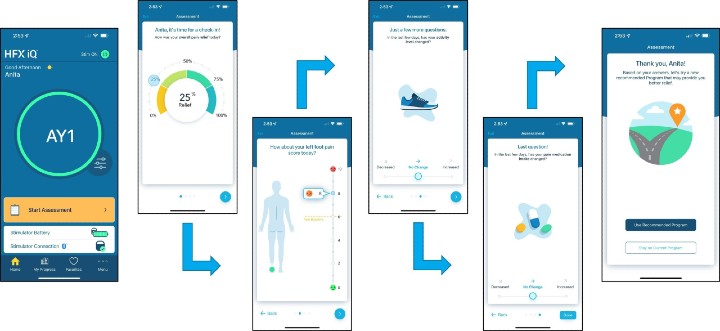
HFX iQ assessments
"HFX iQ is designed to improve the consistency of pain relief and is the only SCS system that truly personalizes care," D. Keith Grossman, chairman and CEO of Nevro told the press. "Pain is variable from patient to patient and over time. Using the big data from our HFX Cloud patient database, our unique HFX Algorithm was developed to identify those programs where patients have been more likely to get relief in the real world. HFX iQ takes direct input from each patient on their pain and quality of life measures to get smarter over time and recommend program changes. HFX iQ utilizes our superior high-frequency 10 kHz Therapy platform, which has been proven in clinical trials and is further supported by real-world evidence from more than 90,000 implanted patients. With HFX iQ, our goal is to provide physicians and patients the personalization needed to achieve and maintain the best possible long-term outcomes. We believe HFX iQ represents the future of SCS therapy."
Nevro will begin limited release in the U.S. this quarter with broader U.S. launch planned for early 2023. The company has also submitted for approval in Europe.
Senza HFX iQ is an artificial intelligence (AI)-based SCS system that learns from patients, developed to address pain variability and help patients optimize and maintain long-term pain relief. The system is composed of the HFX implantable pulse generator (IPG), HFX trial stimulator, charger, and HFX app.
The system will launch with apps tailored for chronic back and leg pain, including non-surgical back pain and painful diabetic neuropathy. Senza HFX iQ was designed to optimize care by collecting data and guiding through a customized treatment pathway. Features include:
- Patients start on the program most likely to provide pain relief, based on the HFX Algorithm built from more than 20 million datapoints and 80,000 implanted patients.
- Recommends customized therapy adjustments informed by patient inputs on the HFX app, including % pain relief, pain score, change in activity levels, and change in pain medications.
- Directly adjusts the pain relief program on the patient's IPG from the HFX app on their smartphone, without the need for a separate manual remote.
- Captures patient and device data to enable sharing of patient-level outcomes with treating and referring physicians.
- Provides a connected, digital environment around a patient's therapy that will allow for future updates to the HFX algorithm, as well as new features and capabilities for patients and providers.

HFX iQ assessments
"HFX iQ is designed to improve the consistency of pain relief and is the only SCS system that truly personalizes care," D. Keith Grossman, chairman and CEO of Nevro told the press. "Pain is variable from patient to patient and over time. Using the big data from our HFX Cloud patient database, our unique HFX Algorithm was developed to identify those programs where patients have been more likely to get relief in the real world. HFX iQ takes direct input from each patient on their pain and quality of life measures to get smarter over time and recommend program changes. HFX iQ utilizes our superior high-frequency 10 kHz Therapy platform, which has been proven in clinical trials and is further supported by real-world evidence from more than 90,000 implanted patients. With HFX iQ, our goal is to provide physicians and patients the personalization needed to achieve and maintain the best possible long-term outcomes. We believe HFX iQ represents the future of SCS therapy."
Nevro will begin limited release in the U.S. this quarter with broader U.S. launch planned for early 2023. The company has also submitted for approval in Europe.













