Michael Barbella, Managing Editor01.31.23
ZimVie Inc. has marked a major milestone with its Mobi-C Cervical Disc, announcing that more than 200,000 discs have been implanted worldwide.
The total includes patients treated in more than 25 countries since the first surgery in France 19 years ago. In 2013, Mobi-C became the first cervical disc to win approval from the U.S. Food and Drug Administration (FDA) for treating more than one level of the cervical spine.
The FDA has determined Mobi-C to be statistically superior to fusion at seven years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial.1 At 10 years, all patient-reported outcomes were equivalent to or improved from seven years.2
“Mobi-C is differentiated by substantial, long-term clinical data and we are proud to announce this milestone, reflecting tens of thousands of patient lives restored with our novel device,” noted Rebecca Whitney, global president of ZimVie Spine. “As we mark the ten-year anniversary of Mobi-C FDA approval for treatment of one and two levels of the cervical spine, we are excited to embrace 2023 as ‘the Year of Mobi-C’ by acknowledging and celebrating throughout the year the clinical success our surgeon customers have realized for their patients.”
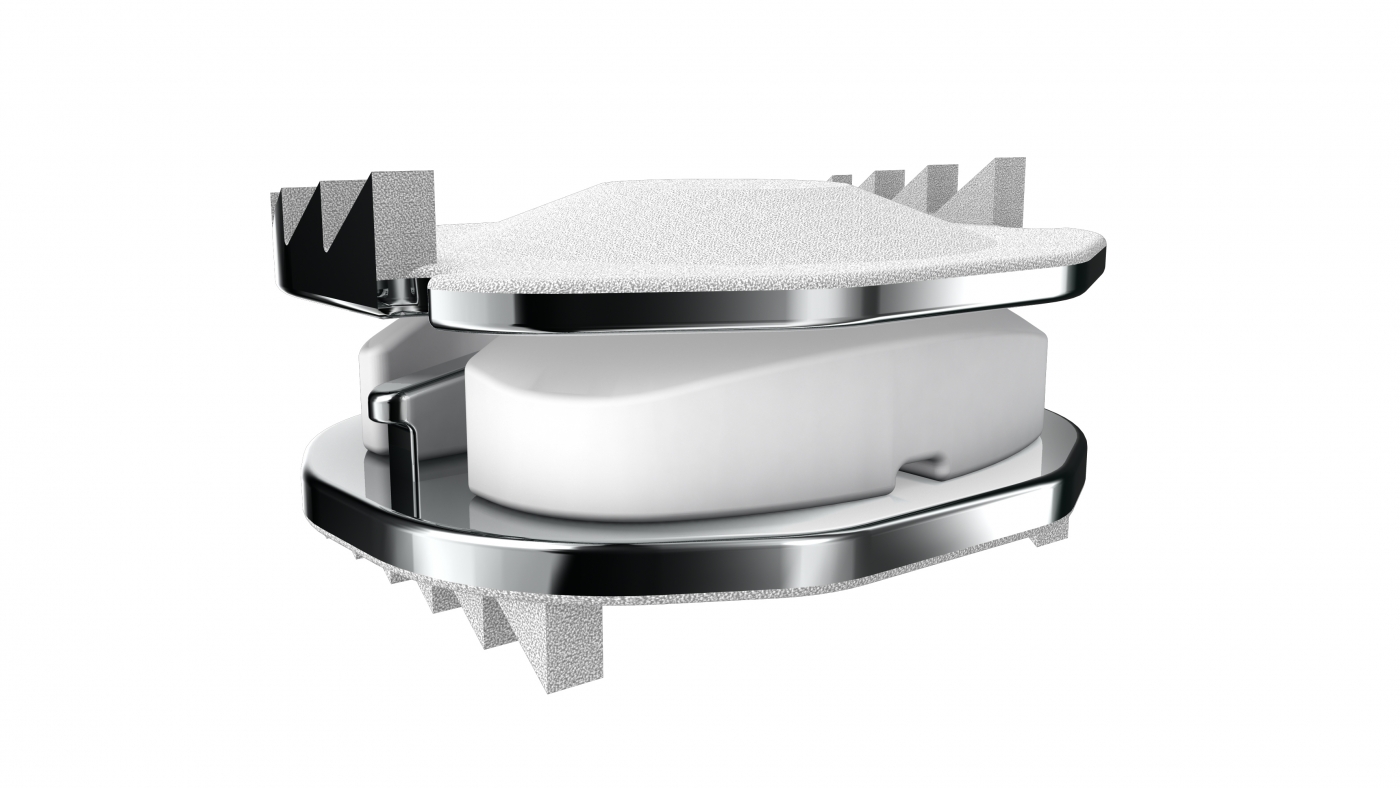
Mobi-C is the first cervical disc prosthesis approved by the FDA for reconstructing a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels. The Mobi-C Cervical Disc Prosthesis is indicated in skeletally mature patients for reconstruction of the disc from C3-C7 following discectomy at one level or two contiguous levels for intractable radiculopathy (arm pain and/or neurological deficit) with or without neck pain or myelopathy due to abnormality localized to the level of the disc space and at least one of the following conditions confirmed by radiographic imaging (CT, MRI or X-rays): herniated nucleus pulposus, spondylosis (defined by the presence of osteophytes) and/or visible loss of disc height compared to adjacent levels. The Mobi-C Cervical Disc Prosthesis is implanted using an anterior approach. Patients should have failed at least six weeks of conservative treatment or demonstrated progressive signs or symptoms despite nonoperative treatment prior to implantation of the Mobi-C Cervical Disc Prosthesis.
“Having participated in the Mobi-C Investigational Device Exemption study over a decade ago, I am encouraged that disc replacement has become an emerging standard of care for patients with cervical disc degeneration. ZimVie’s dedication to this space has enabled so many patients to benefit from the technology. It is fitting to acknowledge this significant milestone for Mobi-C and the advancement of cervical disc arthroplasty," said Dr. Armen Khachatryan, a board-certified orthopedic surgeon at The Disc Replacement Center in West Jordan, Utah. "I sincerely hope that this transition from fusion to motion preserving disc replacement technology will not only continue but accelerate in the coming years.”
Common post-operative risks from surgery with the Mobi-C include pain in the neck, arm, back, shoulder, or head, and dysphagia.
ZimVie develops, manufactures, and delivers a portfolio of products and solutions designed to treat a wide range of spine pathologies and support dental tooth replacement and restoration procedures. The company was founded in March 2022 as an independent, publicly traded spin-off of the Dental and Spine business units of Zimmer Biomet to breathe new life, dedicated energy, and strategic focus to its portfolio of trusted brands and products. From its headquarters in Westminster, Colo., and additional facilities around the globe, the company serves customers in more than 70 countries worldwide.
References
1 Radcliff K, Davis RJ, Hisey MS, et al. Long-term evaluation of cervical disc arthroplasty with the Mobi-C Cervical Disc: a randomized, prospective, multicenter clinical trial with seven-year follow-up. Int J Spine Surg 2017;11(4):244-262. DOI: https://doi.org/10.14444/4031
2 Kim K, Hoffman G, Bae H, et al. Ten-Year Outcomes of 1- and 2-Level Cervical Disc Arthroplasty From the Mobi-C Investigational Device Exemption Clinical Trial. Neurosurgery. 2021;88(3):497-505. DOI: https://doi.org/10.1093/neuros/nyaa459
The total includes patients treated in more than 25 countries since the first surgery in France 19 years ago. In 2013, Mobi-C became the first cervical disc to win approval from the U.S. Food and Drug Administration (FDA) for treating more than one level of the cervical spine.
The FDA has determined Mobi-C to be statistically superior to fusion at seven years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial.1 At 10 years, all patient-reported outcomes were equivalent to or improved from seven years.2
“Mobi-C is differentiated by substantial, long-term clinical data and we are proud to announce this milestone, reflecting tens of thousands of patient lives restored with our novel device,” noted Rebecca Whitney, global president of ZimVie Spine. “As we mark the ten-year anniversary of Mobi-C FDA approval for treatment of one and two levels of the cervical spine, we are excited to embrace 2023 as ‘the Year of Mobi-C’ by acknowledging and celebrating throughout the year the clinical success our surgeon customers have realized for their patients.”
Mobi-C is the first cervical disc prosthesis approved by the FDA for reconstructing a cervical disc at both one and two levels (C3-C7). Mobi-C is a cobalt chromium alloy and polyethylene mobile-bearing prosthesis that is inserted in a single step, without requiring bone chiseling or other vertebral anchorage such as screws or keels. The Mobi-C Cervical Disc Prosthesis is indicated in skeletally mature patients for reconstruction of the disc from C3-C7 following discectomy at one level or two contiguous levels for intractable radiculopathy (arm pain and/or neurological deficit) with or without neck pain or myelopathy due to abnormality localized to the level of the disc space and at least one of the following conditions confirmed by radiographic imaging (CT, MRI or X-rays): herniated nucleus pulposus, spondylosis (defined by the presence of osteophytes) and/or visible loss of disc height compared to adjacent levels. The Mobi-C Cervical Disc Prosthesis is implanted using an anterior approach. Patients should have failed at least six weeks of conservative treatment or demonstrated progressive signs or symptoms despite nonoperative treatment prior to implantation of the Mobi-C Cervical Disc Prosthesis.
“Having participated in the Mobi-C Investigational Device Exemption study over a decade ago, I am encouraged that disc replacement has become an emerging standard of care for patients with cervical disc degeneration. ZimVie’s dedication to this space has enabled so many patients to benefit from the technology. It is fitting to acknowledge this significant milestone for Mobi-C and the advancement of cervical disc arthroplasty," said Dr. Armen Khachatryan, a board-certified orthopedic surgeon at The Disc Replacement Center in West Jordan, Utah. "I sincerely hope that this transition from fusion to motion preserving disc replacement technology will not only continue but accelerate in the coming years.”
Common post-operative risks from surgery with the Mobi-C include pain in the neck, arm, back, shoulder, or head, and dysphagia.
ZimVie develops, manufactures, and delivers a portfolio of products and solutions designed to treat a wide range of spine pathologies and support dental tooth replacement and restoration procedures. The company was founded in March 2022 as an independent, publicly traded spin-off of the Dental and Spine business units of Zimmer Biomet to breathe new life, dedicated energy, and strategic focus to its portfolio of trusted brands and products. From its headquarters in Westminster, Colo., and additional facilities around the globe, the company serves customers in more than 70 countries worldwide.
References
1 Radcliff K, Davis RJ, Hisey MS, et al. Long-term evaluation of cervical disc arthroplasty with the Mobi-C Cervical Disc: a randomized, prospective, multicenter clinical trial with seven-year follow-up. Int J Spine Surg 2017;11(4):244-262. DOI: https://doi.org/10.14444/4031
2 Kim K, Hoffman G, Bae H, et al. Ten-Year Outcomes of 1- and 2-Level Cervical Disc Arthroplasty From the Mobi-C Investigational Device Exemption Clinical Trial. Neurosurgery. 2021;88(3):497-505. DOI: https://doi.org/10.1093/neuros/nyaa459