Michael Barbella, Managing Editor11.16.21
Knee osteoarthritis treatment is getting personal.
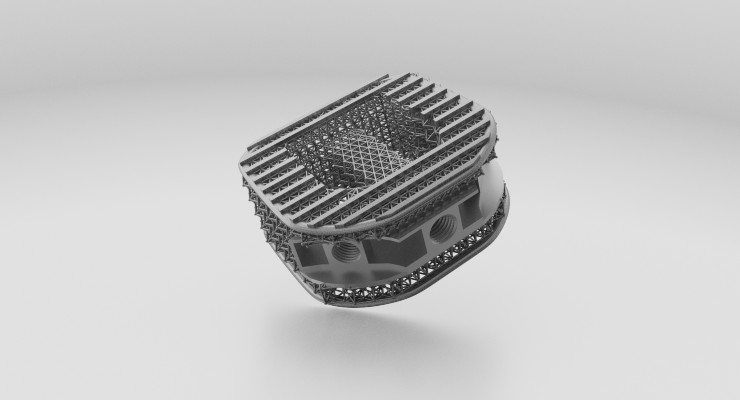
University of Bath (U.K.) researchers have devised a knee realignment system using customized high-tibial osteotomy (HTO) plates made from 3D printed titanium. The plates fit almost perfectly when implanted, thanks to an improved surgical technique also developed by university engineers.
“Knee osteoarthritis is a major health, social, and economic issue, and does not receive as much attention as it should,” said Professor Richie Gill, of the university’s Centre for Therapeutic Innovation. “A quarter of women over 45 have it, and about 15 percent of men, so it’s a significant burden that many live with. Knee replacement is only useful for end-stage osteoarthritis, so you can be in pain and have to live with a disability for a long time, potentially decades, before it’s possible. We hope the new TOKA process we’ve developed will change that.”
Tailored Osteotomy for Knee Alignment (TOKA) aims to improve HTO plate fit and cut OR time four-fold (from two hours to 30 minutes). The procedure uses a 3D CT (computed tomography) scan to create a customized HTO plate and surgical guide that fit the patient’s shin bone as perfectly as a jigsaw puzzle piece. The HTO plates have already been tested in a virtual in-silico trial, and data from the 28 participants convinced U.K. regulators to greenlight a study in Britain. Hospitals in Bath, Bristol, Cardiff, and Exeter are expected to participate in the randomized controlled study to compare patient outcomes with an existing generic HTO procedure.
The TOKA technique also is undergoing testing in Italy, where 25 patients have received customized HTO plates in a trial conducted at the Rizzoli Institute in Bologna.
“The HTO surgery has a long clinical history and it has very good results if done accurately. The difficulty surgeons have is achieving high accuracy, which is why we have created the TOKA method, which starts with a CT scan and digital plan,” Gill said. “3D print the custom knee implant and doing the scanning before operating means surgeons will know exactly what they’ll see before operating and where the implant will go. In addition to a surgeon being able to precisely plan an operation, a surgical guide (or jig) and a plate implant, each personalized to the patient, can be 3D printed automatically based on the scanning data. Importantly, this type of treatment relieves the symptoms of knee osteoarthritis while preserving the natural joint.”
Natural joint preservation and better implant fit are just two of the many advantages of fabricating implants via 3D printing (a.k.a., additive manufacturing). The technology has expanded rapidly in the orthopedic sector in the past decade because it can create more natural anatomical shapes and porous bone replacement scaffolds that allow for natural bone ingrowth, thus ensuring better implant stability.
ODT’s feature “Printer Friendly” explores the ways in which 3D printing is improving orthopedic implant design and patient outcomes. Gaurav Lalwani, medical applications development engineer for Philadelphia-based Carpenter Technology Corporation, was among the half-dozen industry experts interviewed for the story. His full input is provided in the following Q&A.
Michael Barbella: Please discuss the additive manufacturing/3D printing trends currently driving and shaping the orthopedic industry. Have these trends changed of late?
Gaurav Lalwani: Additive manufacturing (AM) has gained widespread adoption in the orthopedic industry. Medical device components such as acetabular cups for total hip arthroplasty (THA) and tibial base plates for total knee arthroplasty (TKA) are routinely printed using electron beam and laser powder bed fusion processes. However, AM has extensively penetrated in the spine subsegment of orthopedics generating positive clinical results. As such, the majority of spinal interbody cages on the market are now produced via AM using titanium material with varying porous structures. Emerging trends such as printing custom patient-specific surgical guides and instrumentation have been explored wherein OEMs don’t need to invest in specialized tooling and subtractive manufacturing processes for low-rate customized production runs. Over the last decade, the technology has matured, and OEMs are exploring next-generation solutions such as printing on hospital site for quick turnaround and targeting challenging applications such as printing femoral stem components for THA, femoral knee component for TKA and expanding interbody cages.
Barbella: What benefits does additive manufacturing bring to the orthopedic industry?
Lalwani: One of the most important reasons for the widespread adoption of AM in orthopedics is that 3D printed implants directly improve patient outcomes. The 3D printed implants with their interconnected porous architectures are designed to improve osseointegration—a key phenomenon to ensure rapid bone healing. This occurs in two phases—during initial surgical implantation, the porous surfaces help to provide initial implant stability and improve host-implant integration. Towards later stages, the 3D printed implants support the adhesion and proliferation of osteoblasts (bone forming cells) into porous channels to produce new bone and accelerate bone healing.
Traditional implants produced via subtractive manufacturing contain solid matrix (non-porous) and are heavy compared to the aforementioned AM implant structural counterpart. These solid high-strength implants typically absorb the routinely experienced physiological loads and do not transfer these loads to the underlying bone—a phenomenon known as stress shielding. If the native bone does not experience mechanical loads due to stress shielding, the bone remodeling process stops and it leads to an increase in osteoclast (bone resorbing cell) activity, making the bones brittle and porous. Implants produced via additive manufacturing have modulus closer to the physiological modulus of the bone and therefore do not induce stress shielding. In addition to providing direct physiological benefits other advantages of AM include the ability of rapid iterative device design from prototype to part enabling the development of patient specific custom surgical guides and implants for complex orthopedic procedures.
Barbella: What challenges are preventing wider scale adoption of additive manufacturing/3D printing in the orthopedic industry?
Lalwani: Although the AM technology and processes have matured over the last decade, several challenges still exist that can potentially limit the adoption of AM.
Not all orthopedic medical device applications are suitable for AM. Although we have witnessed adoption in several subsegments, there are numerous applications that either have design criteria, property requirements or size limitations that can not be met via AM. 3D printing biologically functional coatings remains a challenge.
Barbella: How has additive manufacturing technology impacted/changed orthopedic implant innovation?
Lalwani: Additive manufacturing has accelerated orthopedic implant innovation. AM has enabled the development of patient specific implants and surgical tools. CT and MRI images of patient specific physiology are obtained and reconstructed to enable the production of customized implants and surgical tools rapidly. Additionally, the technologies have provided the design freedom to enable OEMs' selection of more bio-compatible materials. For example, many spine OEMs are now using titanium materials vs. PEEK for interbody devices due to the ability to print cavitation and porous structures that allow for better implant integration without the drawbacks of PEEK (i.e. development of scar tissue around implant). Overall, additive manufacturing is positively influencing patient outcomes due to freedom in design and material selection.
Barbella: How has software enhanced or impacted the design of 3D printed orthopedic implants?
Lalwani: Recent market feedback outlining the need for a more robust powder management and handling solution has led Carpenter to develop PowderLife. This solution provides a combination of products that each play a role in enabling higher productivity and quality in AM by providing traceability from starting feedstock to as-built component. Additionally, easy product recall is possible should a defective powder lot be discovered via simple powder inventory tracking. PowderLife provides safer powder handling, and comprehensive data collection of key process variables (oxygen, temperature, humidity, and pressure). This software and hardware solution allows for efficient management of powder and enables medical OEMs and contract manufacturers to effectively provide validation and traceability information to the regulatory bodies for 510(k) or PMA approvals. We have employed PowderLife in our own AM facilities and continue to evolve its capabilities.
Barbella: What challenges and/or opportunities are associated with using materials other than titanium for 3D printed orthopedic devices/implants and what changes are spurring innovation in orthopedic implants?
Lalwani: Availability of AM optimized non-titanium powders is a positive step to enable widespread adoption of AM beyond the regular use of titanium alloys. For instance, cobalt chrome molybdenum (CCM) alloy is widely used in conventional manufacturing of implants. However, the standard chemistry of CCM results in brittleness and cracking in the AM process. To meet this challenge, Carpenter Technology identified and optimized the trace elemental composition within the ASTM approved limits and developed a CCM powder optimized for 3D printing. Another example of Carpenter’s materials innovation is Ti Grade 23+ powder which provides ~15 percent to 20 percent improvement in mechanical properties vs. standard Ti Grade 23, which enables the design engineers to realize ultra-fine and complex device features that typically fail with regular Ti grade 23 powder. Additionally, availability of next-generation material for AM such as BioDur 108 (Cobalt and nickle-free FDA approved implantable alloy) and Nitinol powders will enable the translation of new applications to AM. The development of new materials needs to be supported with optimized printing parameters to enable successful builds. In addition to material availability, the introduction of multi-laser printers and large format AM platforms is expected to increase throughput and drive lower costs.
Barbella: Where do you see 3D printing in orthopedics headed in the next decade?
Lalwani: Over the next decade we see continuation in the adoption of AM for production of medical devices. We see AM accelerating its evolution from prototyping and low rate production to full-scale production, especially in the spine sub-market. We expect more adoption in other applications such as hips, knees, extremities, and instrumentation. Additionally, the market has just scratched the surface as it relates to understanding the role of powder quality and management on the device effectiveness. Carpenter hopes through its suite of solutions and development of material data sheets to drive this forward and assist in industry evolution.
University of Bath (U.K.) researchers have devised a knee realignment system using customized high-tibial osteotomy (HTO) plates made from 3D printed titanium. The plates fit almost perfectly when implanted, thanks to an improved surgical technique also developed by university engineers.
“Knee osteoarthritis is a major health, social, and economic issue, and does not receive as much attention as it should,” said Professor Richie Gill, of the university’s Centre for Therapeutic Innovation. “A quarter of women over 45 have it, and about 15 percent of men, so it’s a significant burden that many live with. Knee replacement is only useful for end-stage osteoarthritis, so you can be in pain and have to live with a disability for a long time, potentially decades, before it’s possible. We hope the new TOKA process we’ve developed will change that.”
Tailored Osteotomy for Knee Alignment (TOKA) aims to improve HTO plate fit and cut OR time four-fold (from two hours to 30 minutes). The procedure uses a 3D CT (computed tomography) scan to create a customized HTO plate and surgical guide that fit the patient’s shin bone as perfectly as a jigsaw puzzle piece. The HTO plates have already been tested in a virtual in-silico trial, and data from the 28 participants convinced U.K. regulators to greenlight a study in Britain. Hospitals in Bath, Bristol, Cardiff, and Exeter are expected to participate in the randomized controlled study to compare patient outcomes with an existing generic HTO procedure.
The TOKA technique also is undergoing testing in Italy, where 25 patients have received customized HTO plates in a trial conducted at the Rizzoli Institute in Bologna.
“The HTO surgery has a long clinical history and it has very good results if done accurately. The difficulty surgeons have is achieving high accuracy, which is why we have created the TOKA method, which starts with a CT scan and digital plan,” Gill said. “3D print the custom knee implant and doing the scanning before operating means surgeons will know exactly what they’ll see before operating and where the implant will go. In addition to a surgeon being able to precisely plan an operation, a surgical guide (or jig) and a plate implant, each personalized to the patient, can be 3D printed automatically based on the scanning data. Importantly, this type of treatment relieves the symptoms of knee osteoarthritis while preserving the natural joint.”
Natural joint preservation and better implant fit are just two of the many advantages of fabricating implants via 3D printing (a.k.a., additive manufacturing). The technology has expanded rapidly in the orthopedic sector in the past decade because it can create more natural anatomical shapes and porous bone replacement scaffolds that allow for natural bone ingrowth, thus ensuring better implant stability.
ODT’s feature “Printer Friendly” explores the ways in which 3D printing is improving orthopedic implant design and patient outcomes. Gaurav Lalwani, medical applications development engineer for Philadelphia-based Carpenter Technology Corporation, was among the half-dozen industry experts interviewed for the story. His full input is provided in the following Q&A.
Michael Barbella: Please discuss the additive manufacturing/3D printing trends currently driving and shaping the orthopedic industry. Have these trends changed of late?
Gaurav Lalwani: Additive manufacturing (AM) has gained widespread adoption in the orthopedic industry. Medical device components such as acetabular cups for total hip arthroplasty (THA) and tibial base plates for total knee arthroplasty (TKA) are routinely printed using electron beam and laser powder bed fusion processes. However, AM has extensively penetrated in the spine subsegment of orthopedics generating positive clinical results. As such, the majority of spinal interbody cages on the market are now produced via AM using titanium material with varying porous structures. Emerging trends such as printing custom patient-specific surgical guides and instrumentation have been explored wherein OEMs don’t need to invest in specialized tooling and subtractive manufacturing processes for low-rate customized production runs. Over the last decade, the technology has matured, and OEMs are exploring next-generation solutions such as printing on hospital site for quick turnaround and targeting challenging applications such as printing femoral stem components for THA, femoral knee component for TKA and expanding interbody cages.
Barbella: What benefits does additive manufacturing bring to the orthopedic industry?
Lalwani: One of the most important reasons for the widespread adoption of AM in orthopedics is that 3D printed implants directly improve patient outcomes. The 3D printed implants with their interconnected porous architectures are designed to improve osseointegration—a key phenomenon to ensure rapid bone healing. This occurs in two phases—during initial surgical implantation, the porous surfaces help to provide initial implant stability and improve host-implant integration. Towards later stages, the 3D printed implants support the adhesion and proliferation of osteoblasts (bone forming cells) into porous channels to produce new bone and accelerate bone healing.
Traditional implants produced via subtractive manufacturing contain solid matrix (non-porous) and are heavy compared to the aforementioned AM implant structural counterpart. These solid high-strength implants typically absorb the routinely experienced physiological loads and do not transfer these loads to the underlying bone—a phenomenon known as stress shielding. If the native bone does not experience mechanical loads due to stress shielding, the bone remodeling process stops and it leads to an increase in osteoclast (bone resorbing cell) activity, making the bones brittle and porous. Implants produced via additive manufacturing have modulus closer to the physiological modulus of the bone and therefore do not induce stress shielding. In addition to providing direct physiological benefits other advantages of AM include the ability of rapid iterative device design from prototype to part enabling the development of patient specific custom surgical guides and implants for complex orthopedic procedures.
Barbella: What challenges are preventing wider scale adoption of additive manufacturing/3D printing in the orthopedic industry?
Lalwani: Although the AM technology and processes have matured over the last decade, several challenges still exist that can potentially limit the adoption of AM.
Not all orthopedic medical device applications are suitable for AM. Although we have witnessed adoption in several subsegments, there are numerous applications that either have design criteria, property requirements or size limitations that can not be met via AM. 3D printing biologically functional coatings remains a challenge.
- Availability of materials: Majority (more than 95 percent) of the FDA-approved 3D printed medical devices are made of titanium (Ti64ELI). Other alloys such as stainless steels, cobalt chrome molybdenum (CCM), BioDur 108, Nitinol have only recently been available in the marketplace in powder form for 3D printing, they are still in their infancy for adoption in AM.
- Cost – Although 3D printed implants have displayed enhanced physiological performance for certain applications, they generally are not cost competitive with subtractively manufactured conventional implants. Advances in technology such as high throughput multi-laser printing platforms and large format printers are needed to improve operational costs. Additionally, better powder management, reuse and recyclability can also improve operational efficiencies.
- Validated 3D printing capacity and organizational risk – Although the amount of 3D printers have been growing, the validated capacity is still limited. Additional operational capacity needs financial investments with the right “type” of printing platform for specific applications and materials of interest.
- Regulatory uncertainty – FDA has approved several AM devices based on predicate designs under the 510(k) pathway and has recently published a guidance document. However, the document lacks specific details on several AM operational challenges (such as powder reuse, recyclability etc.) and there still exists a lot of uncertainty with the guidance being open for interpretation. Additionally, several next-generation AM materials such as Nitinol, BioDur 108 etc do not have a dedicated ASTM powder specification making it challenging for widespread adoption.
Barbella: How has additive manufacturing technology impacted/changed orthopedic implant innovation?
Lalwani: Additive manufacturing has accelerated orthopedic implant innovation. AM has enabled the development of patient specific implants and surgical tools. CT and MRI images of patient specific physiology are obtained and reconstructed to enable the production of customized implants and surgical tools rapidly. Additionally, the technologies have provided the design freedom to enable OEMs' selection of more bio-compatible materials. For example, many spine OEMs are now using titanium materials vs. PEEK for interbody devices due to the ability to print cavitation and porous structures that allow for better implant integration without the drawbacks of PEEK (i.e. development of scar tissue around implant). Overall, additive manufacturing is positively influencing patient outcomes due to freedom in design and material selection.
Barbella: How has software enhanced or impacted the design of 3D printed orthopedic implants?
Lalwani: Recent market feedback outlining the need for a more robust powder management and handling solution has led Carpenter to develop PowderLife. This solution provides a combination of products that each play a role in enabling higher productivity and quality in AM by providing traceability from starting feedstock to as-built component. Additionally, easy product recall is possible should a defective powder lot be discovered via simple powder inventory tracking. PowderLife provides safer powder handling, and comprehensive data collection of key process variables (oxygen, temperature, humidity, and pressure). This software and hardware solution allows for efficient management of powder and enables medical OEMs and contract manufacturers to effectively provide validation and traceability information to the regulatory bodies for 510(k) or PMA approvals. We have employed PowderLife in our own AM facilities and continue to evolve its capabilities.
Barbella: What challenges and/or opportunities are associated with using materials other than titanium for 3D printed orthopedic devices/implants and what changes are spurring innovation in orthopedic implants?
Lalwani: Availability of AM optimized non-titanium powders is a positive step to enable widespread adoption of AM beyond the regular use of titanium alloys. For instance, cobalt chrome molybdenum (CCM) alloy is widely used in conventional manufacturing of implants. However, the standard chemistry of CCM results in brittleness and cracking in the AM process. To meet this challenge, Carpenter Technology identified and optimized the trace elemental composition within the ASTM approved limits and developed a CCM powder optimized for 3D printing. Another example of Carpenter’s materials innovation is Ti Grade 23+ powder which provides ~15 percent to 20 percent improvement in mechanical properties vs. standard Ti Grade 23, which enables the design engineers to realize ultra-fine and complex device features that typically fail with regular Ti grade 23 powder. Additionally, availability of next-generation material for AM such as BioDur 108 (Cobalt and nickle-free FDA approved implantable alloy) and Nitinol powders will enable the translation of new applications to AM. The development of new materials needs to be supported with optimized printing parameters to enable successful builds. In addition to material availability, the introduction of multi-laser printers and large format AM platforms is expected to increase throughput and drive lower costs.
Barbella: Where do you see 3D printing in orthopedics headed in the next decade?
Lalwani: Over the next decade we see continuation in the adoption of AM for production of medical devices. We see AM accelerating its evolution from prototyping and low rate production to full-scale production, especially in the spine sub-market. We expect more adoption in other applications such as hips, knees, extremities, and instrumentation. Additionally, the market has just scratched the surface as it relates to understanding the role of powder quality and management on the device effectiveness. Carpenter hopes through its suite of solutions and development of material data sheets to drive this forward and assist in industry evolution.