Michael Barbella, Managing Editor02.13.19
Talk about bad timing.
Or in the case of fitness enthusiast Stine Wang Pinilla, truly awful timing.
Pinilla’s terribly inopportune moment occurred 16 months ago as she prepared her stocky 5-foot, 7-inch frame for the 2018 regional CrossFit games. The 31-year-old Danish coach/CrossFit athlete made her inaugural visit to the event in 2017, climbing from 41st in her division to 2nd, and was training hard for a repeat trip when she began experiencing shoulder pain.
With its mix of high-intensity interval training, Olympic weightlifting, plyometrics, powerlifting, gymnastics, girevoy sport, calisthenics, strongman, and other exercises, CrossFit workouts are clearly not for weaklings or weekend gym warriors. The program’s training pushes the body to its absolute limits and sometimes, beyond (Pinilla, for example, can deadlift 325 pounds and back squat 310 pounds); some pain, consequently, is bound to accompany these routines.
Pain has befriended many of Pinilla’s workout regimens over the years, though usually the discomfort has been attributable to minor muscle soreness or overuse.
The pain was different this time, though. There was a deep ache in Pinilla’s shoulder that worsened with even the slightest movement. The underlying issue, Pinilla suspected, was far more serious than muscle fatigue or strain.
She was right: An MRI revealed a rotator cuff injury as well as a previously undetected (but related) cervical spine problem.
“She [Pinilla] was evaluated and it was discovered that not only did she have an injury to three major rotator cuff tendons, but she also had a co-existing cervical spine problem that had never been acknowledged,” recalled John Schultz, M.D., an interventional pain management specialist practicing in Broomfield, Colo. “The two are intimately related.”
Treatment for damaged rotator cuff tendons range from the conservative (icing, rest, physical therapy) and progressive (steroid injections) to the invasive (surgery). Pinilla quickly exhausted the less intrusive options and became an ideal candidate for surgery.
But an operation was out of the question. Average recovery time for rotator cuff procedures is 12-16 weeks, with basic overhead movements like throwing and lifting taking considerably longer (four to six months).
Pinilla didn’t have that kind of time.
“I needed to be ready for the CrossFit open in February [2018], so I knew that surgery and the long recovery that follows was not going to work for me,” she stated.
What eventually worked for Pinilla was, ironically, her body’s natural healing prowess, galvanized by a hearty dose of bone marrow stem cells. She underwent treatment using a superior form of platelet-rich plasma (PRP) to non-surgically repair her damaged rotator cuff tendons and cervical spine muscles. The remedy was designed by Schultz, a regenerative science expert who jointly developed and markets the PRP approach through Des Moines, Iowa-based Regenexx LLC.
Like standard PRP therapies, the Regenexx solution aims to help patients avoid surgery but achieves better outcomes through its bone marrow processing procedure and stem cell recipe, company executives claim.
The secret to Regenexx’s clinical success lies mostly in its PRP processing technique—rather than use a bed- side centrifuge to extract mesenchymal stem cells, the company processes bone marrow in a lab, producing a highly concentrated PRP blend in near-pure form (sans red and white blood cells). Such a mix is optimal for healing orthopedic injuries, according to Regenexx research.
“We utilized precise-guided ultrasound [to inject] high-concentration platelets into the affected areas, both in the rotator cuff and into the cervical spine,” Schultz said in a video posted last spring on the Regenexx web- site. “They [platelets] actually help facilitate healing.”
Indeed, they did: Pinilla received Regenexx stem cell injections on Nov. 14, 2017, and Jan. 11, 2018, and continued to train as she healed. She qualified once again for CrossFit regionals on Feb. 22, 2018.
“In January [2018], I was back to doing everything in my training,” she said. “I’m back to 100 percent and I’m doing all those movements even better than I was before this injury occurred.”
Happy endings like Pinilla’s are adding to a growing body of evidence supporting the efficacy and value of regenerative medicine. Stem cell therapies particularly are resonating with Baby Boomers fighting old age, patients with chronic conditions, hardcore athletes addicted to their sport(s), and a broader American population that is impatient and empowered in managing their healthcare.
Such extensive predilection has spawned a surge in regenerative medicine clinics in recent years. An oft-cited 2016 study counted 570 clinics (more than 85 percent of 351 American businesses) marketing “stem cell” treatments for orthopedic conditions and injuries. That total is now likely closer to 700, with nearly a fifth operating in California, according to the study’s authors.
Not surprisingly, the rise in clinics is abetting developers of stem cell therapies. Regenexx, for example, has licensed its technology to 48 U.S. and international clinics since 2012, and has trained hundreds of doctors in administering its PRP injections. Australian cellular medicine developer Mesoblast Ltd., meanwhile, has logged hundreds of clinical trial hours over the last decade studying biological treatments for spinal conditions, knee osteoarthritis, and general fractures. The company’s most recent trial is examining the efficacy of its allogenic mesenchymal precursor cell product MPC-06-ID in patients with chronic lower back pain due to degenerative disc disease. The 2:1 randomized, placebo-controlled phase 3 trial enrolled 404 patients in 48 centers throughout the United States and Canada. Phase 2 results in 100 patients showed that a single intra-discal injection of MPC-06-ID alleviated pain and improved function for up to three years in patients whose symptoms were not adequately treated with current standard of care therapies.
“[Degenerative disc disease] accounts for approximately 50 percent of opioid prescriptions and is a major contributor to the high annual death rate associated with the ongoing opioid epidemic,” Mesoblast CEO Dr. Silviu Itescu and Chairman Brian Jamieson wrote in the company’s 2018 annual report. “If the phase 3 results confirm the earlier beneficial and sustained effects seen in phase 2 from a single injection of MPC-06-ID on pain and function in these patients, we have the potential to make a major impact on this public health emergency by providing an effective non-opioid alternative.”
Despite all their fantastical potential, however, stem cell therapies like Mesoblast’s MPC-06-ID and Regenexx’s PRP injections are not proven science, and therefore remain largely unsanctioned by the U.S. Food and Drug Administration (FDA). The agency has only approved a handful of umbilical cord blood-based therapies to date (strictly for blood disorders) and is still leery of stem cell injections for any health-related condition.
Part of the reason for the FDA’s skepticism is conflicting study data. A 2016 Mayo Clinic trial, for instance, yielded “uninterpretable” results, showing equivalent outcomes for both stem cell- and saline-treated arthritic knees. Accordingly, researchers deemed stem cell injections a “safe” option for knee pain but not an effective remedy for arthritis.
Regenexx study data, however, refutes the latter deduction. A registry of more than 6,340 same-day knee osteoarthritis patients shows the company’s PRP injections reduced pain levels 48 percent after one month and 57 percent after two years. Stem cells also helped overall joint improvement and function over time, with patients reporting a 54 percent improvement in their knees after six months and a 60 percent improvement after four years. Correspondingly, joint function rose from 71 percent after six months to 78 percent after four years, registry statistics indicate.
Shoulder therapy patients reported similar results: Joint function rose from 66 percent to 82 percent after six months, and climaxed at 88 percent after four years, according to a Regenexx registry of same-day shoulder procedure subjects. Additionally, overall improvement levels more than doubled, going from 29 percent after one month to 72 percent after four years.
The Regenexx data appears to directly contradict a 2018 Rush University study that rendered PRP therapy largely ineffective at healing major rotator cuff damage. The analysis concluded that stem cells work best in a supplemental role (for small and medium-sized tears and in high-risk patients), as they “have not been shown to improve healing rates or patient-reported outcomes in large level one studies,” researchers wrote. “Mesenchymal stem cells have been shown to improve healing rates but not functional outcomes, though high-level human trials are lacking.”
Those kinds of trials may not be lacking for long, though. FDA scientists currently are working to identify cell therapy product characteristics that could predict the performance reliability of cell-based human therapies. Moreover, the agency last year gave the Mayo Clinic permission to operate an automated bioreactor-based stem cell production platform on its Jacksonville, Fla., campus. The approval essentially enables the Mayo Clinic Center for Regenerative Medicine to produce a large amount of bone marrow stem cells for clinical trial use.
“Although the Mayo Clinic has been poised to scale up regenerative clinical trials, to date we did not have the capacity to support them,” Abba Zubair, M.D., Ph.D., medical director of Transfusion Medicine and the Human Cell Therapy Laboratory (Florida campus), said last January. “With this new technology, we can now develop phase II trials enrolling larger numbers of patients to fully test the efficacy of cell-based therapies.”
Certainly, more sophisticated, larger-scale testing of stem cell therapies will improve the safety and efficacy of products, but it also will spawn a better balance of consumer protection and medical innovation, industry experts assert.
“The number one factor in clinicians’ and industry professionals’ mind is not only patient outcomes but also a long-term safety record: allowing the patient’s body to heal itself in an optimized environment, preventing recurrences, and restoring full function,” noted Tyler Richardson, director of marketing, Surgical Biologics, at AMNIOX Medical Inc., a subsidiary of regenerative amniotic tissue-based product developer TissueTech Inc. “Any time foreign material is placed in the body, there is a concern of what the patient’s response to that material will be with respect to rejection. That’s why using an immune-privileged biologic source like human placental tissue with devitalized cells is advantageous.”
Its advantages are numerous, actually: Amniotic mesenchymal stem cells (MSCs)—gathered from the placenta immediately after childbirth—guard against infection; naturally reduce pain, inflammation, and scarring; and are impervious to human immune system attacks. Additionally, amniotic membranes contain high amounts of cytokines and growth factors—the two ingredients essential for cell migration and procreation.
Those same fixings are present in umbilical cord tissue, too. Otherwise known as Wharton’s Jelly, umbilical cord MSCs increasingly are being used to treat such orthopedic conditions as muscle tears, bone injuries, and herniated discs.
Case in point: AMNIOX Medical’s umbilical cord/amniotic membrane matrix product, RESPINA, has been especially effective in improving microdiscectomy outcomes. Clinical trial results show the matrix improved function, reduced pain and inflammation, and curtailed recurrent disc herniations. In addition, RESPINA-treated patients reported superior post-surgery functionality at six weeks and 24 months compared to standard care (no tissue implant).
The company’s CLARIX 100 (cryopreserved placental tissue) worked equally as well for discectomy outcomes. Like RESPINA, the regenerative matrix successfully hampered disc reherniations, reduced pain, and improved daily functionality as early as six weeks post-surgery. Patients treated only with cryopreserved umbilical cord tissue responded even better to treatment, AMNIOX Medical reported.
“The company’s proprietary cryopreservation method to retain significantly greater amounts of key effector proteins and growth factors, cytokines, and the HC-HA/PTX3 complex, has the potential to facilitate regenerative healing and help manage functional recovery,” Richardson said. “Promoting regenerative healing and incorporation may help reduce surgical complications and have positive downstream health economic effects.”
Extremely positive, in fact. Studies have shown that umbilical cord/amniotic membrane (UC/AM) therapy is a more cost-effective treatment for fractures, as it can reduce complications and minimize repeat procedures. In heel bone injuries, UC/AM treatment has led to a 10 percent decrease in overall complications, and a 5 percent dip in hospital readmissions, according to trial data published last summer in Today’s Wound Clinic.
Comparable results ensued with complicated open and closed fractures. In a 33-patient study where UC/AM was administered as an adjunctive wound therapy, only 7 percent of subjects needed additional operations and 10 percent underwent procedures to control infection, data showed. None of the patients, however, needed plastic surgery to close their wounds.
“Building on several years of strong performance, orthobiologic products have come of age, proving to be more effective in many instances at repairing injured or defective tissues than other types of medical devices,” explained Ryanne Early, CEO of biological material-derived product manufacturer Seed Biotech Inc. The Dallas, Texas-based firm’s two products—an acellular allograft amniotic membrane (Aril) and acellular placental tissue particulate (Petil) are designed for use in orthopedic, surgical, ophthalmologic, and spinal applications.
“Continued success of well-known product lines has led surgeons to realize the potential of a wide range of biologic products available for accelerating tissue healing,” Early continued. “The continuous improvement in the safety and supply of tissue products and continued innovation in orthobiologic products will be necessary to address [market] needs.”
Those needs are not expected to change much over the next two decades as the planet’s population matures and succumbs to the crippling conditions associated with deteriorating bones and muscles. Degenerative disc disease disorders, for example, are expected to impact at least 130 million people globally by 2050, with knee and hip osteoarthritis emerging as the worst offenders, according to Arthritis Foundation projections.
Sports-related injuries, traumas, and demand for minimally invasive procedures are expected to shape the sector as well, transforming orthobiologics into a $6.5 billion global juggernaut by 2027, Future Market Insights estimates. Bone graft stimulators will remain the largest industry subsector, ballooning to roughly $3 billion in value, followed by viscosupplements, which is forecast to grow more than 3.5 percent annually over the next eight years.
“The key drivers of innovation are the increasing number of elderly people suffering from orthopedic degenerative disc diseases, the use of regenerative therapies in sports medicine, technological advancements and R&D, and orthopedic device companies moving into the regenerative medicine space,” said Jeff Sauter, director of business development for Steri-Tek, a drug/device manufacturer based in Fremont, Calif. “Demand has surged for minimally invasive procedures such as bone grafting and growth factor treatment.”
Bone grafts foster healing through a variety of osteo-conductive, osteoinductive, and osteogenic mechanisms. Autografts are generally the preferable treatment option, since they are sourced directly from the patient and pose little, if any, threat of disease transmission or biological rejection. Donor bone (allograft) eliminates the need for a second procedure (harvest surgery) and spares expenses, but it poses a greater risk of rejection and (host) disease transmission.
The risks associated with allografts have helped fuel interest in and demand for bone graft substitutes. Alter- natives include demineralized bone matrixes (DBMs), ceramics, coral, graft composites, and bone morphogenetic proteins (naturally-occurring substances that regulate bone formation and healing). These proteins have been clinically proven to expedite healing and limit adverse reactions to donor bone and non-bone alternatives.
“There is an increased awareness and attention on synthetic osteoconductive scaffold innovations. There is growing demand for synthetic bone grafting substitutes that provide better handling properties for physicians, aiding in ease-of-use and making bone grafting more efficient,” declared Clay Shors, Ph.D., president of Biogennix, an Irvine, Calif.-based developer of osteobiological products for posterolateral spine and long bone fusion. “Bone graft substitutes that are biocompatible, resorbable, and aid in enhancing efficiency and results are sought after right now.”
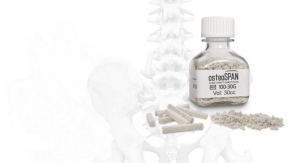
Among those in-demand solutions is Biogennix’s osteoSPAN and Morpheus resorbable scaffolds. The calcium phosphate-calcium carbonate composites—used to treat more than 11,000 patients—have complete interconnected porosity (65 percent) and an optimal pore diameter for bone reconstruction, approximately 500 microns (µ).
osteoSPAN is most effective when used as a bone graft extender in posterior lumbar spine fusion, according to study data. A 60-patient trial linked the product’s resorption rate to the degree of bone fusion, showing resorption increasing simultaneously with bone formation (presumably due to interplay between osteoclasts and osteoblasts).
Morpheus works equally as well in posterolateral spinal fusions, but it also is effective in pelvic and extremity procedures. The product is a moldable form of osteoSPAN granules, composed of 1-2 mm grains suspended in a rapidly resorbable organic binder to facilitate implant placement and containment. The synthetic polymer binder provides improved intraoperative handling characteristics over granules alone and does not interfere with osteoSPAN’s osteoconductive properties, Morpheus product literature states. During clinical use, the bone graft left less residual material on surgical gloves, making it easier for surgeons to hold medical instruments.
“Due to the increased average age of the population, as well as lifestyle factors, the rate of spinal fusion procedures has risen rapidly over the past several decades,”Shors said. “In addition, as structural and biologic reconstructive solutions continue to be improved and demonstrate procedural success, more patients seek these treatments, more surgeons dedicate their practices to these treatments, further contributing to the growth cycle. With close to half a million spinal fusion procedures performed each year and growing, spinal fusion appears to be the dominating orthobiologics application.”
And that dominance is likely to continue for the foreseeable future due to market drivers like aging spines, technological advancements (expendable interbody cages, navigation systems), and the growing popularity of minimally invasive surgical techniques. Industry statistics forecast the global spinal fusion sector to rise 3.4 percent annually through 2023 to reach almost $9 billion, with spinal biologics surging 4.4 percent yearly to exceed $2.5 billion.
Those growth projections are helping drive innovation in the market and sparking solutions that provide clinical value to both patients and payers. An increasing number of these technologies are coming from large OEMs that once were strictly focused on joint and fracture fixation.
Stryker Corp., for example, now provides a line of bone grafts (Vitoss) and demineralized bone matrix (BIO), as well as a bone marrow aspirate system (Imbibe) while Zimmer Biomet Holding Inc. manufactures a bone graft substitute (Pro Osteon), bone void filler (CopiOs) DFMs (Puros and InterGro), and a bone graft matrix (PlatFORM).
NuVasive Inc.’s orthobiologics portfolio, however, is more focused on smart biomaterials for advanced surface technologies. The market need is driving innovation in the company’s orthobiologics and spinal implant hardware offerings.
“To support better bone fusion outcomes for patients, the company is focused on the development of complementary orthobiologics products, with AttraX Scaffold representing one of our latest disruptive biologics portfolio additions,” said Albert Cornejo, head of Biologics at San Diego, Calif.-based NuVasive. “This ceramic bone graft offers a microarchitecture and microporosity optimized for bone formation.”
AttraX Scaffold is an absorbent ceramic-collagen bone graft with an optimized surface that has been validated in clinical testing to drive increased bone formation and faster fusion than traditional ceramic grafts.
Unlike traditional ceramic materials that do not by themselves generate bone formation when implanted in an intramuscular site, NuVasive’s AttraX Scaffold has a microstructure and microporosity that are optimized for bone formation in this environment. The product’s surface technology, through its carefully defined and tightly controlled features at the submicron scale, drives the differentiation of mesenchymal stem cells into bone-forming osteoblasts without added growth factors.
Available in strips, blocks and morsels, AttraX Scaffold can be used in the posterolateral spine to promote fusion. During preclinical testing in posterolateral fusion (PLF) models, AttraX fusion rates were equivalent to or better than autograft, and faster than traditional ceramic grafts. In addition, spinal segments fused with AttraX had greater biomechanical strength than segments treated with ACTIFUSE ABX or Vitoss BA in a rabbit PLF model.
“Aligning with our beliefs that smart biomaterials with advanced surface technology represent a key trend in the orthobiologics market, AttraX Scaffold is one of the newest additions we’re currently focused on, as it represents a disruption of the ceramic bone graft substitute space,” Cornejo noted. “NuVasive believes surface optimization represents the next leap forward in ceramic bone grafting technology with promising potential to deliver better clinical outcomes for surgeons and their patients.
Or in the case of fitness enthusiast Stine Wang Pinilla, truly awful timing.
Pinilla’s terribly inopportune moment occurred 16 months ago as she prepared her stocky 5-foot, 7-inch frame for the 2018 regional CrossFit games. The 31-year-old Danish coach/CrossFit athlete made her inaugural visit to the event in 2017, climbing from 41st in her division to 2nd, and was training hard for a repeat trip when she began experiencing shoulder pain.
With its mix of high-intensity interval training, Olympic weightlifting, plyometrics, powerlifting, gymnastics, girevoy sport, calisthenics, strongman, and other exercises, CrossFit workouts are clearly not for weaklings or weekend gym warriors. The program’s training pushes the body to its absolute limits and sometimes, beyond (Pinilla, for example, can deadlift 325 pounds and back squat 310 pounds); some pain, consequently, is bound to accompany these routines.
Pain has befriended many of Pinilla’s workout regimens over the years, though usually the discomfort has been attributable to minor muscle soreness or overuse.
The pain was different this time, though. There was a deep ache in Pinilla’s shoulder that worsened with even the slightest movement. The underlying issue, Pinilla suspected, was far more serious than muscle fatigue or strain.
She was right: An MRI revealed a rotator cuff injury as well as a previously undetected (but related) cervical spine problem.
“She [Pinilla] was evaluated and it was discovered that not only did she have an injury to three major rotator cuff tendons, but she also had a co-existing cervical spine problem that had never been acknowledged,” recalled John Schultz, M.D., an interventional pain management specialist practicing in Broomfield, Colo. “The two are intimately related.”
Treatment for damaged rotator cuff tendons range from the conservative (icing, rest, physical therapy) and progressive (steroid injections) to the invasive (surgery). Pinilla quickly exhausted the less intrusive options and became an ideal candidate for surgery.
But an operation was out of the question. Average recovery time for rotator cuff procedures is 12-16 weeks, with basic overhead movements like throwing and lifting taking considerably longer (four to six months).
Pinilla didn’t have that kind of time.
“I needed to be ready for the CrossFit open in February [2018], so I knew that surgery and the long recovery that follows was not going to work for me,” she stated.
What eventually worked for Pinilla was, ironically, her body’s natural healing prowess, galvanized by a hearty dose of bone marrow stem cells. She underwent treatment using a superior form of platelet-rich plasma (PRP) to non-surgically repair her damaged rotator cuff tendons and cervical spine muscles. The remedy was designed by Schultz, a regenerative science expert who jointly developed and markets the PRP approach through Des Moines, Iowa-based Regenexx LLC.
Like standard PRP therapies, the Regenexx solution aims to help patients avoid surgery but achieves better outcomes through its bone marrow processing procedure and stem cell recipe, company executives claim.
The secret to Regenexx’s clinical success lies mostly in its PRP processing technique—rather than use a bed- side centrifuge to extract mesenchymal stem cells, the company processes bone marrow in a lab, producing a highly concentrated PRP blend in near-pure form (sans red and white blood cells). Such a mix is optimal for healing orthopedic injuries, according to Regenexx research.
“We utilized precise-guided ultrasound [to inject] high-concentration platelets into the affected areas, both in the rotator cuff and into the cervical spine,” Schultz said in a video posted last spring on the Regenexx web- site. “They [platelets] actually help facilitate healing.”
Indeed, they did: Pinilla received Regenexx stem cell injections on Nov. 14, 2017, and Jan. 11, 2018, and continued to train as she healed. She qualified once again for CrossFit regionals on Feb. 22, 2018.
“In January [2018], I was back to doing everything in my training,” she said. “I’m back to 100 percent and I’m doing all those movements even better than I was before this injury occurred.”
Happy endings like Pinilla’s are adding to a growing body of evidence supporting the efficacy and value of regenerative medicine. Stem cell therapies particularly are resonating with Baby Boomers fighting old age, patients with chronic conditions, hardcore athletes addicted to their sport(s), and a broader American population that is impatient and empowered in managing their healthcare.
Such extensive predilection has spawned a surge in regenerative medicine clinics in recent years. An oft-cited 2016 study counted 570 clinics (more than 85 percent of 351 American businesses) marketing “stem cell” treatments for orthopedic conditions and injuries. That total is now likely closer to 700, with nearly a fifth operating in California, according to the study’s authors.
Not surprisingly, the rise in clinics is abetting developers of stem cell therapies. Regenexx, for example, has licensed its technology to 48 U.S. and international clinics since 2012, and has trained hundreds of doctors in administering its PRP injections. Australian cellular medicine developer Mesoblast Ltd., meanwhile, has logged hundreds of clinical trial hours over the last decade studying biological treatments for spinal conditions, knee osteoarthritis, and general fractures. The company’s most recent trial is examining the efficacy of its allogenic mesenchymal precursor cell product MPC-06-ID in patients with chronic lower back pain due to degenerative disc disease. The 2:1 randomized, placebo-controlled phase 3 trial enrolled 404 patients in 48 centers throughout the United States and Canada. Phase 2 results in 100 patients showed that a single intra-discal injection of MPC-06-ID alleviated pain and improved function for up to three years in patients whose symptoms were not adequately treated with current standard of care therapies.
“[Degenerative disc disease] accounts for approximately 50 percent of opioid prescriptions and is a major contributor to the high annual death rate associated with the ongoing opioid epidemic,” Mesoblast CEO Dr. Silviu Itescu and Chairman Brian Jamieson wrote in the company’s 2018 annual report. “If the phase 3 results confirm the earlier beneficial and sustained effects seen in phase 2 from a single injection of MPC-06-ID on pain and function in these patients, we have the potential to make a major impact on this public health emergency by providing an effective non-opioid alternative.”
|
Tim Reed, Marketing Director, Strategic Alliances, DePuy Synthes In today’s environment, biomaterials must be effective as customers are striving to reduce OR time and get patients ambulatory and out of care facilities sooner. Continuing to meet the goals of the Triple Aim remains vital for the medical devices industry—improving health outcomes while reducing treatment costs and enhancing the patient experience—remains critical. Biomaterials can play an important role in helping to achieve these goals. Biomaterials are very frequently used in spine fusion procedures as well as in many other orthopedic surgeries, such as extremity cases and trauma procedures. Surgeons are often interested in working with DePuy Synthes and Johnson & Johnson Medical Devices Companies—manufacturers that can provide a full range of hardware fixation products and biomaterials solutions for all orthopedic and neurological spine surgeries across the continuum of care. Using bone graft substitutes instead of harvesting autograft from the patient’s iliac crest or operative surgical site epitomizes the industry’s commitment to improving health outcomes and the overall patient experience. ViviGen Cellular Bone Matrix, a differentiated cellular allograft indicated for repairing or reconstructing musculoskeletal defects that DePuy Synthes distributes in collaboration with LifeNet Health, is a product that can be used in an effort to help achieve these goals. On a more micro-level, DePuy Synthes is seeing customers continue to demand less costly alternatives to bone morphogenetic protein (BMP)-based products, driven by price sensitivity . DePuy Synthes recently added the FIBERGRAFT Technology family of products to its portfolio, a next-generation line of synthetic bone graft materials that are ultra-porous, designed for ease of use, and have been engineered for optimal resorption during spine fusion and trauma procedures. By adding FIBERGRAFT Technology to its offerings, the company is further enhancing its biomaterials portfolio that currently includes cellular allograft, demineralized bone matrix, and first-generation synthetic solutions. ViviGen and FIBERGRAFT Technology are solutions that represent categories of alternatives to recombinant protein-based products. Surgeons now have a broad range of technologies and handling options in the area of biomaterials based on the needs of each patient and surgical case. On the customer education side, the challenge remains in helping customers navigate the myriad of products available while ensuring DePuy Synthes’ portfolio includes solutions that can be cost-effective and be used in surgeries and cases that vary in levels of complexity and challenges. There are many different brands of bone void filler products on the market today, ranging from simple allograft chips and ceramic granules to highly engineered cellular allografts, bioactive ceramics, and BMPs. It is very challenging for customers to know the differences between products, why some are more expensive, and why certain products may be better suited for different procedures. As a leader in professional education, DePuy Synthes strives to reduce this complexity by making biomaterials an integral part of its curriculum across all orthopedic specialties and ensuring that all the company’s offerings have well-defined value propositions with accompanying explanations that can directly highlight the clinical benefits of each product. Biomaterials will continue to be important in helping to drive healing for patients and DePuy Synthes will work to identify ways to bring new innovations to market in this critical area. ViviGen is a registered trademark of LifeNet Health. FIBERGRAFT is owned by Prosidyan. 106279-190125 DSUS |
Despite all their fantastical potential, however, stem cell therapies like Mesoblast’s MPC-06-ID and Regenexx’s PRP injections are not proven science, and therefore remain largely unsanctioned by the U.S. Food and Drug Administration (FDA). The agency has only approved a handful of umbilical cord blood-based therapies to date (strictly for blood disorders) and is still leery of stem cell injections for any health-related condition.
Part of the reason for the FDA’s skepticism is conflicting study data. A 2016 Mayo Clinic trial, for instance, yielded “uninterpretable” results, showing equivalent outcomes for both stem cell- and saline-treated arthritic knees. Accordingly, researchers deemed stem cell injections a “safe” option for knee pain but not an effective remedy for arthritis.
Regenexx study data, however, refutes the latter deduction. A registry of more than 6,340 same-day knee osteoarthritis patients shows the company’s PRP injections reduced pain levels 48 percent after one month and 57 percent after two years. Stem cells also helped overall joint improvement and function over time, with patients reporting a 54 percent improvement in their knees after six months and a 60 percent improvement after four years. Correspondingly, joint function rose from 71 percent after six months to 78 percent after four years, registry statistics indicate.
Shoulder therapy patients reported similar results: Joint function rose from 66 percent to 82 percent after six months, and climaxed at 88 percent after four years, according to a Regenexx registry of same-day shoulder procedure subjects. Additionally, overall improvement levels more than doubled, going from 29 percent after one month to 72 percent after four years.
The Regenexx data appears to directly contradict a 2018 Rush University study that rendered PRP therapy largely ineffective at healing major rotator cuff damage. The analysis concluded that stem cells work best in a supplemental role (for small and medium-sized tears and in high-risk patients), as they “have not been shown to improve healing rates or patient-reported outcomes in large level one studies,” researchers wrote. “Mesenchymal stem cells have been shown to improve healing rates but not functional outcomes, though high-level human trials are lacking.”
Those kinds of trials may not be lacking for long, though. FDA scientists currently are working to identify cell therapy product characteristics that could predict the performance reliability of cell-based human therapies. Moreover, the agency last year gave the Mayo Clinic permission to operate an automated bioreactor-based stem cell production platform on its Jacksonville, Fla., campus. The approval essentially enables the Mayo Clinic Center for Regenerative Medicine to produce a large amount of bone marrow stem cells for clinical trial use.
“Although the Mayo Clinic has been poised to scale up regenerative clinical trials, to date we did not have the capacity to support them,” Abba Zubair, M.D., Ph.D., medical director of Transfusion Medicine and the Human Cell Therapy Laboratory (Florida campus), said last January. “With this new technology, we can now develop phase II trials enrolling larger numbers of patients to fully test the efficacy of cell-based therapies.”
Certainly, more sophisticated, larger-scale testing of stem cell therapies will improve the safety and efficacy of products, but it also will spawn a better balance of consumer protection and medical innovation, industry experts assert.
“The number one factor in clinicians’ and industry professionals’ mind is not only patient outcomes but also a long-term safety record: allowing the patient’s body to heal itself in an optimized environment, preventing recurrences, and restoring full function,” noted Tyler Richardson, director of marketing, Surgical Biologics, at AMNIOX Medical Inc., a subsidiary of regenerative amniotic tissue-based product developer TissueTech Inc. “Any time foreign material is placed in the body, there is a concern of what the patient’s response to that material will be with respect to rejection. That’s why using an immune-privileged biologic source like human placental tissue with devitalized cells is advantageous.”
Its advantages are numerous, actually: Amniotic mesenchymal stem cells (MSCs)—gathered from the placenta immediately after childbirth—guard against infection; naturally reduce pain, inflammation, and scarring; and are impervious to human immune system attacks. Additionally, amniotic membranes contain high amounts of cytokines and growth factors—the two ingredients essential for cell migration and procreation.
Those same fixings are present in umbilical cord tissue, too. Otherwise known as Wharton’s Jelly, umbilical cord MSCs increasingly are being used to treat such orthopedic conditions as muscle tears, bone injuries, and herniated discs.
Case in point: AMNIOX Medical’s umbilical cord/amniotic membrane matrix product, RESPINA, has been especially effective in improving microdiscectomy outcomes. Clinical trial results show the matrix improved function, reduced pain and inflammation, and curtailed recurrent disc herniations. In addition, RESPINA-treated patients reported superior post-surgery functionality at six weeks and 24 months compared to standard care (no tissue implant).
The company’s CLARIX 100 (cryopreserved placental tissue) worked equally as well for discectomy outcomes. Like RESPINA, the regenerative matrix successfully hampered disc reherniations, reduced pain, and improved daily functionality as early as six weeks post-surgery. Patients treated only with cryopreserved umbilical cord tissue responded even better to treatment, AMNIOX Medical reported.
“The company’s proprietary cryopreservation method to retain significantly greater amounts of key effector proteins and growth factors, cytokines, and the HC-HA/PTX3 complex, has the potential to facilitate regenerative healing and help manage functional recovery,” Richardson said. “Promoting regenerative healing and incorporation may help reduce surgical complications and have positive downstream health economic effects.”
Extremely positive, in fact. Studies have shown that umbilical cord/amniotic membrane (UC/AM) therapy is a more cost-effective treatment for fractures, as it can reduce complications and minimize repeat procedures. In heel bone injuries, UC/AM treatment has led to a 10 percent decrease in overall complications, and a 5 percent dip in hospital readmissions, according to trial data published last summer in Today’s Wound Clinic.
Comparable results ensued with complicated open and closed fractures. In a 33-patient study where UC/AM was administered as an adjunctive wound therapy, only 7 percent of subjects needed additional operations and 10 percent underwent procedures to control infection, data showed. None of the patients, however, needed plastic surgery to close their wounds.
“Building on several years of strong performance, orthobiologic products have come of age, proving to be more effective in many instances at repairing injured or defective tissues than other types of medical devices,” explained Ryanne Early, CEO of biological material-derived product manufacturer Seed Biotech Inc. The Dallas, Texas-based firm’s two products—an acellular allograft amniotic membrane (Aril) and acellular placental tissue particulate (Petil) are designed for use in orthopedic, surgical, ophthalmologic, and spinal applications.
“Continued success of well-known product lines has led surgeons to realize the potential of a wide range of biologic products available for accelerating tissue healing,” Early continued. “The continuous improvement in the safety and supply of tissue products and continued innovation in orthobiologic products will be necessary to address [market] needs.”
Those needs are not expected to change much over the next two decades as the planet’s population matures and succumbs to the crippling conditions associated with deteriorating bones and muscles. Degenerative disc disease disorders, for example, are expected to impact at least 130 million people globally by 2050, with knee and hip osteoarthritis emerging as the worst offenders, according to Arthritis Foundation projections.
Sports-related injuries, traumas, and demand for minimally invasive procedures are expected to shape the sector as well, transforming orthobiologics into a $6.5 billion global juggernaut by 2027, Future Market Insights estimates. Bone graft stimulators will remain the largest industry subsector, ballooning to roughly $3 billion in value, followed by viscosupplements, which is forecast to grow more than 3.5 percent annually over the next eight years.
“The key drivers of innovation are the increasing number of elderly people suffering from orthopedic degenerative disc diseases, the use of regenerative therapies in sports medicine, technological advancements and R&D, and orthopedic device companies moving into the regenerative medicine space,” said Jeff Sauter, director of business development for Steri-Tek, a drug/device manufacturer based in Fremont, Calif. “Demand has surged for minimally invasive procedures such as bone grafting and growth factor treatment.”
Bone grafts foster healing through a variety of osteo-conductive, osteoinductive, and osteogenic mechanisms. Autografts are generally the preferable treatment option, since they are sourced directly from the patient and pose little, if any, threat of disease transmission or biological rejection. Donor bone (allograft) eliminates the need for a second procedure (harvest surgery) and spares expenses, but it poses a greater risk of rejection and (host) disease transmission.
The risks associated with allografts have helped fuel interest in and demand for bone graft substitutes. Alter- natives include demineralized bone matrixes (DBMs), ceramics, coral, graft composites, and bone morphogenetic proteins (naturally-occurring substances that regulate bone formation and healing). These proteins have been clinically proven to expedite healing and limit adverse reactions to donor bone and non-bone alternatives.
“There is an increased awareness and attention on synthetic osteoconductive scaffold innovations. There is growing demand for synthetic bone grafting substitutes that provide better handling properties for physicians, aiding in ease-of-use and making bone grafting more efficient,” declared Clay Shors, Ph.D., president of Biogennix, an Irvine, Calif.-based developer of osteobiological products for posterolateral spine and long bone fusion. “Bone graft substitutes that are biocompatible, resorbable, and aid in enhancing efficiency and results are sought after right now.”
Among those in-demand solutions is Biogennix’s osteoSPAN and Morpheus resorbable scaffolds. The calcium phosphate-calcium carbonate composites—used to treat more than 11,000 patients—have complete interconnected porosity (65 percent) and an optimal pore diameter for bone reconstruction, approximately 500 microns (µ).
osteoSPAN is most effective when used as a bone graft extender in posterior lumbar spine fusion, according to study data. A 60-patient trial linked the product’s resorption rate to the degree of bone fusion, showing resorption increasing simultaneously with bone formation (presumably due to interplay between osteoclasts and osteoblasts).
Morpheus works equally as well in posterolateral spinal fusions, but it also is effective in pelvic and extremity procedures. The product is a moldable form of osteoSPAN granules, composed of 1-2 mm grains suspended in a rapidly resorbable organic binder to facilitate implant placement and containment. The synthetic polymer binder provides improved intraoperative handling characteristics over granules alone and does not interfere with osteoSPAN’s osteoconductive properties, Morpheus product literature states. During clinical use, the bone graft left less residual material on surgical gloves, making it easier for surgeons to hold medical instruments.
“Due to the increased average age of the population, as well as lifestyle factors, the rate of spinal fusion procedures has risen rapidly over the past several decades,”Shors said. “In addition, as structural and biologic reconstructive solutions continue to be improved and demonstrate procedural success, more patients seek these treatments, more surgeons dedicate their practices to these treatments, further contributing to the growth cycle. With close to half a million spinal fusion procedures performed each year and growing, spinal fusion appears to be the dominating orthobiologics application.”
And that dominance is likely to continue for the foreseeable future due to market drivers like aging spines, technological advancements (expendable interbody cages, navigation systems), and the growing popularity of minimally invasive surgical techniques. Industry statistics forecast the global spinal fusion sector to rise 3.4 percent annually through 2023 to reach almost $9 billion, with spinal biologics surging 4.4 percent yearly to exceed $2.5 billion.
Those growth projections are helping drive innovation in the market and sparking solutions that provide clinical value to both patients and payers. An increasing number of these technologies are coming from large OEMs that once were strictly focused on joint and fracture fixation.
Stryker Corp., for example, now provides a line of bone grafts (Vitoss) and demineralized bone matrix (BIO), as well as a bone marrow aspirate system (Imbibe) while Zimmer Biomet Holding Inc. manufactures a bone graft substitute (Pro Osteon), bone void filler (CopiOs) DFMs (Puros and InterGro), and a bone graft matrix (PlatFORM).
NuVasive Inc.’s orthobiologics portfolio, however, is more focused on smart biomaterials for advanced surface technologies. The market need is driving innovation in the company’s orthobiologics and spinal implant hardware offerings.
“To support better bone fusion outcomes for patients, the company is focused on the development of complementary orthobiologics products, with AttraX Scaffold representing one of our latest disruptive biologics portfolio additions,” said Albert Cornejo, head of Biologics at San Diego, Calif.-based NuVasive. “This ceramic bone graft offers a microarchitecture and microporosity optimized for bone formation.”
AttraX Scaffold is an absorbent ceramic-collagen bone graft with an optimized surface that has been validated in clinical testing to drive increased bone formation and faster fusion than traditional ceramic grafts.
Unlike traditional ceramic materials that do not by themselves generate bone formation when implanted in an intramuscular site, NuVasive’s AttraX Scaffold has a microstructure and microporosity that are optimized for bone formation in this environment. The product’s surface technology, through its carefully defined and tightly controlled features at the submicron scale, drives the differentiation of mesenchymal stem cells into bone-forming osteoblasts without added growth factors.
Available in strips, blocks and morsels, AttraX Scaffold can be used in the posterolateral spine to promote fusion. During preclinical testing in posterolateral fusion (PLF) models, AttraX fusion rates were equivalent to or better than autograft, and faster than traditional ceramic grafts. In addition, spinal segments fused with AttraX had greater biomechanical strength than segments treated with ACTIFUSE ABX or Vitoss BA in a rabbit PLF model.
“Aligning with our beliefs that smart biomaterials with advanced surface technology represent a key trend in the orthobiologics market, AttraX Scaffold is one of the newest additions we’re currently focused on, as it represents a disruption of the ceramic bone graft substitute space,” Cornejo noted. “NuVasive believes surface optimization represents the next leap forward in ceramic bone grafting technology with promising potential to deliver better clinical outcomes for surgeons and their patients.