Michael Barbella, Managing Editor05.23.19
The Bird is grounded again. And for quite a while this time, too.
Few in the New York Yankees organization were surprised by first baseman Greg Bird’s move to the injured list in mid-April. The 26-year-old, after all, has been plagued by injuries since turning professional eight years ago, and has never played a full season with the 27-time World Series champions. MLB data show Bird has played in only 186 regular season games with the team over the past four years and has missed more than 370 due to shoulder, foot, elbow, and ankle ailments.
“Unfortunately, it is a little bit of his story so far, not being fully healthy,” Yankees Manager Aaron Boone said on April 16 after placing Bird on the injured list with a left plantar fascia tear. “Even the times he’s been able to play through things, they’ve held him back to a point. I still fully believe he [Bird] can be an impact hitter in this league, but you’ve got to stay healthy to be able to do that. And that’s been the biggest challenge. We’ll continue to try to find a way to help him be at his healthiest all the time and hopefully we get to a point where that happens.”
The Yankees won’t get to that point this season: Bird is out at least through May 27 as he waits for his foot to heal. The good news is Bird won’t need surgery (the tear in the connective tissue spanning the bottom of the foot usually heals well on its own) but the bad news is the average healing time for plantar fascia tears is 12 weeks. That means Bird could potentially miss half the season.
Once more.
Perhaps more important than the timetable for Bird’s return, however, is the company he’s keeping while recuperating. Roughly a dozen Yankees currently are sidelined along with Bird, nursing injuries ranging from strained calves and sore backs to inflamed shoulders and surgically repaired elbows. Center fielder Jacoby Ellsbury is Bird’s kindred spirit among the Yankees’ walking wounded, as he also is recovering from plantar fasciitis.
“It’s crazy,” first baseman Luke Voit (Bird’s replacement) told The New York Times after an April 28 game against the San Francisco Giants. “It’s like we have a new face in our locker room every day.”
One of those new faces was infield sub D.J. LeMahieu, who left that Giants game with a bruised right knee from a foul ball two days earlier. “It’s been the story of the season...” he mused to the Times.
It’s also a story that could have lasting impacts. Though many of the injuries vexing the Yankee lineup this season are relatively minor (small-joint strains, sprains, tightness, inflammation), they possibly could lead to more substantial problems in the future. Ankle sprains, for example, can beget re-injury (often in the same spot), joint damage, chronic instability, and ACL (anterior cruciate ligament) troubles. Human and animal studies, in fact, suggest the effects of a sprained ankle can be more substantial and lingering than previously thought, potentially altering the ability and frequency of movement for life.
One analysis showed lower activity levels among college students with past sprains, while other studies involving mice chronicled a lifetime of fewer movements among the injured rodents.
Despite the parallels, though, researchers were hesitant to link the studies’ conclusions (mice and mortals are so very different, they noted). Nevertheless, the data proves that ankle sprains should not be taken lightly.
“The ankle is the base of the body,” study leader Tricia Hubbard-Turner, kinesiology professor at UNC-Charlotte, told the Times. “Don’t ignore a sprain.”
Consider yourself warned, Clint Frazier.
Fortunately, the Yankees outfielder is not without options should the Grade 2 ankle sprain he suffered last month haunt him in the future. Stabilization help is available through Arthrex Inc.’s InternalBrace, a seatbelt-like device that allows bruised ligaments to heal physiologically, or the Talus HemiCAP, a joint restoration system designed to precisely match a patient’s cartilage surface. Developed by Franklin, Mass.-based Arthrosurface, the HemiCAP implant consists of a screw that pairs with a contoured cap covering the damaged cartilage (and healthy tissue as well); the system, according to the company, aims to prevent further joint damage while maintaining the patient’s native anatomy and motion.
Other future ankle options for Frazier include Wright Medical Group N.V.’s GRAFTJACKET regenerative tissue matrix and BIOFIBER CM, a collagen-coated, resorbable, biologically-derived fiber scaffold for tendon repair.
More alternatives are likely to surface the longer Frazier (a.k.a., “Red Thunder”) and his teammates play professional baseball. A steady increase in sports-related injuries, combined with rising osteoporosis rates and the planet’s aging population, is projected to boost the global foot and ankle segment a market-leading 8.7 percent annually through 2025, industry data indicate. The potential for new discoveries and innovations also is expected to fuel growth.
“The reason foot and ankle is the fastest-growing [extremity] segment is because there is still so much research to do,” Paragon 28 co-founder, president, and CEO Albert DaCosta noted in a Business in Focus article last spring. “Whereas with the knee, there are fewer procedures to consider, with the foot we have hundreds of techniques for addressing just a bunion deformity. There are several large segments like bunion deformities, fracture fixation, and flat foot reconstruction; within each of these categories there are hundreds of procedures for us to consider and improve.”
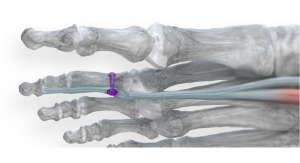
Such considerations and improvements have led to dozens of innovations since the company’s 2010 birth, including treatments for flat feet, bunions, and hammertoe. Paragon 28’s newest addition to the latter product portfolio is the TenoTac soft tissue fixation system, a product that aims to both streamline procedures and give surgeons more flexibility in their technique and approach to hammertoe issues.
Launched in February, the TenoTac system uses a titanium threaded implant and a simple insertion method to obtain optimal fixation of soft tissue to bone. Temporary fixation occurs once the implant is inserted and fixation takes place when the dorsal and plantar teeth bite into the bone. The implant is made in three sizes to fit different patient anatomies and comes with minimal instruments to provide a less invasive solution for tendon transfers.
Paragon 28 claims TenoTac embodies a unique surgical approach compared to traditional methods for flexor tendon transfers, as it corrects the deformity without releasing tissue attachments distally by balancing plantar and dorsal tension.
“We are creating specific products for each indication and each area of the foot, with consideration of blood supply, soft tissue coverage, and weight-bearing requirements for that area,” DaCosta explained to Business in Focus. “That is how we are creating technologies specific to the foot.”
That’s how other companies are creating solutions, too. Extremity Medical has developed a cannulated PEEK hammertoe device for osteotomy fixation and reconstructing the lesser toes after corrective procedures for hammertoe, claw toe, and mallet toe. Similarly, Memphis, Tenn.-based CrossRoads Extremity Systems has devised a line of implants for treating forefoot, midfoot, and rearfoot conditions (DynaFORCE), along with devices for Plantar Plate repair and hammertoe deformity.
Additionally, market newcomer Nvision Biomedical Technologies—which formerly focused only on spinal implants—is now testing the foot/ankle sector waters with its PEEK-OPTIMA hammertoe correction system. The San Antonio, Texas-headquartered firm claims its Vector system is the first U.S. Food and Drug Administration (FDA)-approved foot and ankle implant made from PEEK-OPTIMA, and is the first lower extremity device to use Structural Encoding to enable the FDA-required Unique Device Identification.
Hammertoe and bunion correction solutions are portfolio staples at Additive Orthopaedics LLC, Centric Medical, In2Bones Global Inc., and Novastep Inc. as well, but these firms are also striving for distinction through innovation or anatomical defect. In2Bones opted for the latter choice, developing a soft tissue release system (ClearGuard) that enables surgeons to minimally invasively treat various conditions, including gastrocnemius strains/tears, tarsal tunnel syndrome, Morton’s neuroma, and plantar fasciitis.
Take heed, Greg Bird and Jacoby Ellsbury.
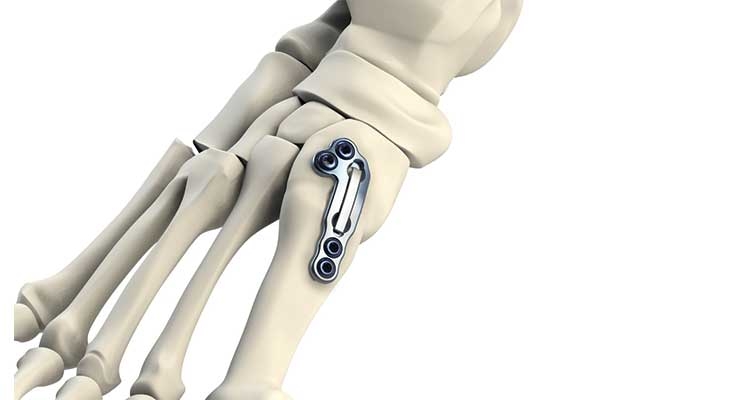
Centric Medical, Novastep, and Additive Orthopaedics, meanwhile, are gunning for prominence via technology: Centric manufactures a customized external fixator (SATURN); Novastep sells size-specific, pre-shaped allograft bone wedges (biofit); and Additive Orthopaedics is leveraging one of the industry’s hottest trends to make bone wedges, bone segments, and patient-specific (3D-printed) locking lattice plates that ultimately will become the cornerstone of an integrated surgical planning system the company hopes to launch later this year.
“There will always be a need for traditional off-the-shelf orthopedic devices, but in cases of implant revisions, limb salvage, and trauma, using printing to manufacture a patient-specific device can potentially lead to a better outcome,” Additive Orthopaedics president Greg Kowalczyk said upon the FDA’s mid-January approval of the company’s lattice plates. “Although we are still in the early stages for 3D printed patient-specific implants, it has been a terrific journey developing the market in foot and ankle orthopaedics.”
While that market shows promise, it likely will be years (possibly decades) before its value approaches that of the overall foot/ankle sector, estimated to be worth more than $6.46 billion within the next six years. Growth most certainly will come from the proliferation of age- and lifestyle-related conditions (osteoarthritis and diabetes, particularly) as well as product and technological advancements that simplify procedures, reduce hospitalization, and provide quality patient care.
“Growth is being driven by innovations that make procedures easier, more predictable to perform, and more efficient for the healthcare system,” stated Julie Dewey, senior vice president and chief communications officer at Wright Medical Group. “Lower extremities growth is being driven by end-stage ankle arthritis, specifically total ankle replacement, as well as innovative implants that keep patients moving. There is also growing interest around minimally invasive solutions that get patients back on their feet quicker with less pain and an improved cosmetic result.”
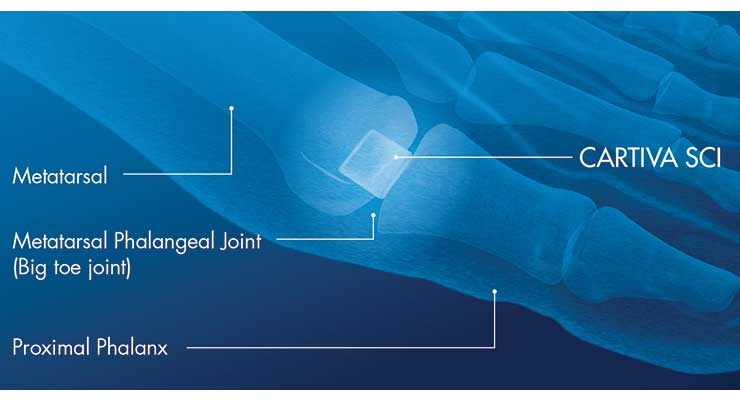
Wright Medical is aiding patient recovery and movement with its PROstep MIS System and Cartiva Synthetic Cartilage Implant, the latter of which was inherited through the company’s $435 million acquisition of Cartiva Inc. last summer. The artificial cartilage implant is used as an alternative to fusion to reduce pain and maintain motion in the big toe joint. It is the only PMA-approved product for treating great toe osteoarthritis, according to the firm.
Composed of a biocompatible, durable, low-friction organic polymer, Cartiva reduces joint pain without sacrificing the foot’s natural movement, thereby enabling patients to retain their mobility and range of motion.
Clinical studies have linked Cartiva to durable pain relief, sustainable functional improvement, and a better range of motion (a 25 percent increase) compared with fusion.
The PROstep MIS also has been clinically proven to improve outcomes, with studies showing the product to significantly reduce perioperative pain, shorten hospital stays, and minimize the risk of wound complications.
Developed specifically for foot and ankle surgery, the PROstep system provides a minimally invasive (MI), procedurally integrated solution that features specially designed implants and instruments for percutaneous foot surgery. The PROstep System is used to treat various forefoot and hindfoot conditions, including bunions (one of the most common foot deformities).
The MI surgery is performed through small 3-5mm incisions rather than the 3- to 5-inch cuts used in standard bunion surgery. Osteotomies also are less invasive with the PROstep System, as doctors use a rotating burr tool instead of a micro saggital saw.
In addition to solutions like PROstep and Cartiva, Wright Medical is helping improve patient outcomes and control healthcare costs through its preoperative planning technology, an innovation that has become more of a competitive tool in the era of value-based medicine. Most orthopedic implant makers have developed such systems in recent years as they strive to improve procedural accuracy and better treat musculoskeletal conditions.
Wright Medical’s PROPHECY Preoperative Navigation System for total ankle replacements allows doctors to visualize patients’ anatomies and anticipate potential surgical contingencies before the actual procedure. Using a computed tomography (CT) scan, the system models a patient’s defective joint and simulates the replacement surgery, helping engineers craft a better-fitting implant. A study of ankle replacement surgeries using the PROPHECY Navigation System found the tibial component size to be accurate in 98 percent of cases and alignment to be within 5 degrees or less of the target in 100 percent of subjects.
Indeed, the PROPHECY system offers multiple benefits to both patients and caregivers: Patients receive a longer-lasting, more anatomically compatible joint (which lowers the risk of revision surgery), while caregivers reduce OR time through a streamlined procedure (which ultimately saves hospitals money). A win-win for all parties, certainly.
“Surgical planning is here to stay,” Dewey told Orthopedic Design & Technology. “We believe this is the future of orthopedic healthcare, and we believe we are well-positioned to be a market leader with software platforms both now and in the future.”
Wright Medical is attempting to ensure its future market status with the establishment of a new digital division that will oversee the company’s digital surgery strategy. Announced in early May, the division is tasked with developing new platforms, accelerating surgical solutions, and driving software tech across the business.
The division will build upon the company’s existing BLUEPRINT 3D planning software, which allows surgeons to simulate the position of a shoulder prosthesis using CT imaging. The software provides doctors with a three-dimensional view of the shoulder as well as the ability to digitally rotate through the complete range of motion in any direction in order to choose the best possible implant for patients.
“We have committed ourselves to the development of our BLUEPRINT software platform that will deliver not only surgical planning but the creation of a digital ecosystem that has value elements for all of the stakeholders involved in patient care,” Dewey noted. “We believe that software-enabled solutions like BLUEPRINT are the future.”
Many of Wright Medical’s rivals share that philosophy as well: Exactech Inc. has devised software that instantly reconstructs the scapula in 3D, and Zimmer Biomet Holdings Inc. sells a preoperative planning platform that creates “pinpoint” accurate patient-specific surgical guides for anatomic and reverse shoulder arthroplasty candidates.
Both techniques are the focus of a Web platform developed by DePuy Synthes and Materialise NV to improve the digital planning, design, and ordering of patient-specific 3D printed guides for shoulder surgery. The platform debuted last spring at the American Academy of Orthopaedic Surgeons annual meeting.
“...this collaboration will empower more orthopaedic surgeons to discover the benefits of online planning...” Materialise founder/CEO Wilfried Vancraen declared at the time of the launch. “3D planning is gaining popularity with orthopedic surgeons performing shoulder operations.”
And that popularity is bound to grow over the next half-decade as more geriatrics seek to avoid infirmity and more athletes look to bypass (or briefly visit) injured lists.
ResearchAndMarkets.com data estimate the worldwide shoulder replacement market to grow 7.6 percent annually over the next five years to reach $1.9 billion. Potential headwinds during the forecast period include postoperative complications and implant costs.
“Patient needs will drive the development of new devices. As the market share grows for shoulder arthroplasty, revisions will become more frequent due to volume and the active aging population,” said Baptiste Martin, CEO of FX Shoulder USA Inc., the Dallas, Texas-based subsidiary of French firm FX Solutions S.A.S., that provides and distributes shoulder replacement devices in the United States. “There is a trend to follow revisions, particularly in cases involving bone loss of the glenoid, and to develop specific prostheses to treat patients and (potentially) improve outcomes and post-operative range of motion.”
FX Shoulder is focusing on the arthroplasty trend with its FDA-cleared Humeris and Humelock product portfolios; the Humelock II and Humelock Reversed are offered with optional screws (depending on surgeons’ preference for cement). FX Solutions also offers a stemless device (Easytech) for patients with arthritis and good bone quality, but it is now only approved in Europe.
The company, however, is attempting to expand its use across the pond, having launched in December 2018 an FDA-approved Investigational Device Exemption clinical study of the Easytech Reversed stemless shoulder prosthesis. Twelve U.S. surgeons at nine sites are participating in the trial.
“The Easytech Reversed may be exactly what the U.S. market is in need of,” Martin said, “and a reversed stemless prosthesis could potentially be the future of shoulder arthroplasty.”
It certainly seems that way. Market research shows a growing use of stemless implants in shoulder arthroplasty as surgeons seek to simplify revision procedures and prevent stem-related complications. Doctors also are finding an incentive for use through the increasing number of clinical studies proving the efficacy of stemless implants. A September 2017 study demonstrated nine-year clinical outcome comparability of stemless shoulder devices to that of third- and fourth-generation standard shoulder arthroplasty, as well as significant improvements in patient satisfaction.
Stemless implants are superficially similar to a resurfacing except for their thicker metaphyseal anchorage and larger component head. Contrary to their name, these devices have a short stem, but it does not enter the diaphysis of the bone like a stemmed implant; rather, it remains completely in the humeral neck mataphysis.
Despite its growing popularity, stemless arthroplasty is more prominent in Europe than the United States. Only a handful of these solutions are currently available in America, including Arthrex’s Eclipse, Wright Medical’s Simpliciti, Zimmer Biomet’s Sidus Stem-Free Shoulder, and Arthrosurface’s OVO Primary Stemless Shoulder System with Inlay Glenoid.
Arthrosurface’s OVO device was approved by the FDA last spring and first implanted in August. Touted as the only ovoid (egg)-shaped implant on the market, the OVO helps surgeons avoid overstuffing the joint, improves patients’ range of motion, and reduces pain. Moreover, the prosthesis uses a taper post that secures into dense, subchondral bone for strong fixation, and the inlay glenoid implant significantly reduces glenoid loosening due to the elimination of “rocking horse” effect.
“The shoulder market is exciting in the sheer volume of procedures, and the continued adoption and improvement of high-performance shoulder arthroplasty devices,” Arthrosurface CEO Steve Ek said. “The market now has many examples of devices that are entering the second and third generations of their designs. Therefore, I think we are starting to see more consistent and reproducible results with these devices. At the same time, patients and surgeons are starting to demand a greater level of performance and function from these devices. Where once the goal was just to mitigate pain, now I believe the expectation is the presumed elimination of pain but also the restoration of a high level of function.”
Care to verify that, Greg Bird?
Few in the New York Yankees organization were surprised by first baseman Greg Bird’s move to the injured list in mid-April. The 26-year-old, after all, has been plagued by injuries since turning professional eight years ago, and has never played a full season with the 27-time World Series champions. MLB data show Bird has played in only 186 regular season games with the team over the past four years and has missed more than 370 due to shoulder, foot, elbow, and ankle ailments.
“Unfortunately, it is a little bit of his story so far, not being fully healthy,” Yankees Manager Aaron Boone said on April 16 after placing Bird on the injured list with a left plantar fascia tear. “Even the times he’s been able to play through things, they’ve held him back to a point. I still fully believe he [Bird] can be an impact hitter in this league, but you’ve got to stay healthy to be able to do that. And that’s been the biggest challenge. We’ll continue to try to find a way to help him be at his healthiest all the time and hopefully we get to a point where that happens.”
The Yankees won’t get to that point this season: Bird is out at least through May 27 as he waits for his foot to heal. The good news is Bird won’t need surgery (the tear in the connective tissue spanning the bottom of the foot usually heals well on its own) but the bad news is the average healing time for plantar fascia tears is 12 weeks. That means Bird could potentially miss half the season.
Once more.
Perhaps more important than the timetable for Bird’s return, however, is the company he’s keeping while recuperating. Roughly a dozen Yankees currently are sidelined along with Bird, nursing injuries ranging from strained calves and sore backs to inflamed shoulders and surgically repaired elbows. Center fielder Jacoby Ellsbury is Bird’s kindred spirit among the Yankees’ walking wounded, as he also is recovering from plantar fasciitis.
“It’s crazy,” first baseman Luke Voit (Bird’s replacement) told The New York Times after an April 28 game against the San Francisco Giants. “It’s like we have a new face in our locker room every day.”
One of those new faces was infield sub D.J. LeMahieu, who left that Giants game with a bruised right knee from a foul ball two days earlier. “It’s been the story of the season...” he mused to the Times.
It’s also a story that could have lasting impacts. Though many of the injuries vexing the Yankee lineup this season are relatively minor (small-joint strains, sprains, tightness, inflammation), they possibly could lead to more substantial problems in the future. Ankle sprains, for example, can beget re-injury (often in the same spot), joint damage, chronic instability, and ACL (anterior cruciate ligament) troubles. Human and animal studies, in fact, suggest the effects of a sprained ankle can be more substantial and lingering than previously thought, potentially altering the ability and frequency of movement for life.
One analysis showed lower activity levels among college students with past sprains, while other studies involving mice chronicled a lifetime of fewer movements among the injured rodents.
Despite the parallels, though, researchers were hesitant to link the studies’ conclusions (mice and mortals are so very different, they noted). Nevertheless, the data proves that ankle sprains should not be taken lightly.
“The ankle is the base of the body,” study leader Tricia Hubbard-Turner, kinesiology professor at UNC-Charlotte, told the Times. “Don’t ignore a sprain.”
Consider yourself warned, Clint Frazier.
Fortunately, the Yankees outfielder is not without options should the Grade 2 ankle sprain he suffered last month haunt him in the future. Stabilization help is available through Arthrex Inc.’s InternalBrace, a seatbelt-like device that allows bruised ligaments to heal physiologically, or the Talus HemiCAP, a joint restoration system designed to precisely match a patient’s cartilage surface. Developed by Franklin, Mass.-based Arthrosurface, the HemiCAP implant consists of a screw that pairs with a contoured cap covering the damaged cartilage (and healthy tissue as well); the system, according to the company, aims to prevent further joint damage while maintaining the patient’s native anatomy and motion.
Other future ankle options for Frazier include Wright Medical Group N.V.’s GRAFTJACKET regenerative tissue matrix and BIOFIBER CM, a collagen-coated, resorbable, biologically-derived fiber scaffold for tendon repair.
More alternatives are likely to surface the longer Frazier (a.k.a., “Red Thunder”) and his teammates play professional baseball. A steady increase in sports-related injuries, combined with rising osteoporosis rates and the planet’s aging population, is projected to boost the global foot and ankle segment a market-leading 8.7 percent annually through 2025, industry data indicate. The potential for new discoveries and innovations also is expected to fuel growth.
“The reason foot and ankle is the fastest-growing [extremity] segment is because there is still so much research to do,” Paragon 28 co-founder, president, and CEO Albert DaCosta noted in a Business in Focus article last spring. “Whereas with the knee, there are fewer procedures to consider, with the foot we have hundreds of techniques for addressing just a bunion deformity. There are several large segments like bunion deformities, fracture fixation, and flat foot reconstruction; within each of these categories there are hundreds of procedures for us to consider and improve.”
Such considerations and improvements have led to dozens of innovations since the company’s 2010 birth, including treatments for flat feet, bunions, and hammertoe. Paragon 28’s newest addition to the latter product portfolio is the TenoTac soft tissue fixation system, a product that aims to both streamline procedures and give surgeons more flexibility in their technique and approach to hammertoe issues.
Launched in February, the TenoTac system uses a titanium threaded implant and a simple insertion method to obtain optimal fixation of soft tissue to bone. Temporary fixation occurs once the implant is inserted and fixation takes place when the dorsal and plantar teeth bite into the bone. The implant is made in three sizes to fit different patient anatomies and comes with minimal instruments to provide a less invasive solution for tendon transfers.
Paragon 28 claims TenoTac embodies a unique surgical approach compared to traditional methods for flexor tendon transfers, as it corrects the deformity without releasing tissue attachments distally by balancing plantar and dorsal tension.
“We are creating specific products for each indication and each area of the foot, with consideration of blood supply, soft tissue coverage, and weight-bearing requirements for that area,” DaCosta explained to Business in Focus. “That is how we are creating technologies specific to the foot.”
That’s how other companies are creating solutions, too. Extremity Medical has developed a cannulated PEEK hammertoe device for osteotomy fixation and reconstructing the lesser toes after corrective procedures for hammertoe, claw toe, and mallet toe. Similarly, Memphis, Tenn.-based CrossRoads Extremity Systems has devised a line of implants for treating forefoot, midfoot, and rearfoot conditions (DynaFORCE), along with devices for Plantar Plate repair and hammertoe deformity.
Additionally, market newcomer Nvision Biomedical Technologies—which formerly focused only on spinal implants—is now testing the foot/ankle sector waters with its PEEK-OPTIMA hammertoe correction system. The San Antonio, Texas-headquartered firm claims its Vector system is the first U.S. Food and Drug Administration (FDA)-approved foot and ankle implant made from PEEK-OPTIMA, and is the first lower extremity device to use Structural Encoding to enable the FDA-required Unique Device Identification.
Hammertoe and bunion correction solutions are portfolio staples at Additive Orthopaedics LLC, Centric Medical, In2Bones Global Inc., and Novastep Inc. as well, but these firms are also striving for distinction through innovation or anatomical defect. In2Bones opted for the latter choice, developing a soft tissue release system (ClearGuard) that enables surgeons to minimally invasively treat various conditions, including gastrocnemius strains/tears, tarsal tunnel syndrome, Morton’s neuroma, and plantar fasciitis.
Take heed, Greg Bird and Jacoby Ellsbury.
Centric Medical, Novastep, and Additive Orthopaedics, meanwhile, are gunning for prominence via technology: Centric manufactures a customized external fixator (SATURN); Novastep sells size-specific, pre-shaped allograft bone wedges (biofit); and Additive Orthopaedics is leveraging one of the industry’s hottest trends to make bone wedges, bone segments, and patient-specific (3D-printed) locking lattice plates that ultimately will become the cornerstone of an integrated surgical planning system the company hopes to launch later this year.
“There will always be a need for traditional off-the-shelf orthopedic devices, but in cases of implant revisions, limb salvage, and trauma, using printing to manufacture a patient-specific device can potentially lead to a better outcome,” Additive Orthopaedics president Greg Kowalczyk said upon the FDA’s mid-January approval of the company’s lattice plates. “Although we are still in the early stages for 3D printed patient-specific implants, it has been a terrific journey developing the market in foot and ankle orthopaedics.”
While that market shows promise, it likely will be years (possibly decades) before its value approaches that of the overall foot/ankle sector, estimated to be worth more than $6.46 billion within the next six years. Growth most certainly will come from the proliferation of age- and lifestyle-related conditions (osteoarthritis and diabetes, particularly) as well as product and technological advancements that simplify procedures, reduce hospitalization, and provide quality patient care.
“Growth is being driven by innovations that make procedures easier, more predictable to perform, and more efficient for the healthcare system,” stated Julie Dewey, senior vice president and chief communications officer at Wright Medical Group. “Lower extremities growth is being driven by end-stage ankle arthritis, specifically total ankle replacement, as well as innovative implants that keep patients moving. There is also growing interest around minimally invasive solutions that get patients back on their feet quicker with less pain and an improved cosmetic result.”
Wright Medical is aiding patient recovery and movement with its PROstep MIS System and Cartiva Synthetic Cartilage Implant, the latter of which was inherited through the company’s $435 million acquisition of Cartiva Inc. last summer. The artificial cartilage implant is used as an alternative to fusion to reduce pain and maintain motion in the big toe joint. It is the only PMA-approved product for treating great toe osteoarthritis, according to the firm.
Composed of a biocompatible, durable, low-friction organic polymer, Cartiva reduces joint pain without sacrificing the foot’s natural movement, thereby enabling patients to retain their mobility and range of motion.
Clinical studies have linked Cartiva to durable pain relief, sustainable functional improvement, and a better range of motion (a 25 percent increase) compared with fusion.
The PROstep MIS also has been clinically proven to improve outcomes, with studies showing the product to significantly reduce perioperative pain, shorten hospital stays, and minimize the risk of wound complications.
Developed specifically for foot and ankle surgery, the PROstep system provides a minimally invasive (MI), procedurally integrated solution that features specially designed implants and instruments for percutaneous foot surgery. The PROstep System is used to treat various forefoot and hindfoot conditions, including bunions (one of the most common foot deformities).
The MI surgery is performed through small 3-5mm incisions rather than the 3- to 5-inch cuts used in standard bunion surgery. Osteotomies also are less invasive with the PROstep System, as doctors use a rotating burr tool instead of a micro saggital saw.
In addition to solutions like PROstep and Cartiva, Wright Medical is helping improve patient outcomes and control healthcare costs through its preoperative planning technology, an innovation that has become more of a competitive tool in the era of value-based medicine. Most orthopedic implant makers have developed such systems in recent years as they strive to improve procedural accuracy and better treat musculoskeletal conditions.
Wright Medical’s PROPHECY Preoperative Navigation System for total ankle replacements allows doctors to visualize patients’ anatomies and anticipate potential surgical contingencies before the actual procedure. Using a computed tomography (CT) scan, the system models a patient’s defective joint and simulates the replacement surgery, helping engineers craft a better-fitting implant. A study of ankle replacement surgeries using the PROPHECY Navigation System found the tibial component size to be accurate in 98 percent of cases and alignment to be within 5 degrees or less of the target in 100 percent of subjects.
Indeed, the PROPHECY system offers multiple benefits to both patients and caregivers: Patients receive a longer-lasting, more anatomically compatible joint (which lowers the risk of revision surgery), while caregivers reduce OR time through a streamlined procedure (which ultimately saves hospitals money). A win-win for all parties, certainly.
“Surgical planning is here to stay,” Dewey told Orthopedic Design & Technology. “We believe this is the future of orthopedic healthcare, and we believe we are well-positioned to be a market leader with software platforms both now and in the future.”
Wright Medical is attempting to ensure its future market status with the establishment of a new digital division that will oversee the company’s digital surgery strategy. Announced in early May, the division is tasked with developing new platforms, accelerating surgical solutions, and driving software tech across the business.
The division will build upon the company’s existing BLUEPRINT 3D planning software, which allows surgeons to simulate the position of a shoulder prosthesis using CT imaging. The software provides doctors with a three-dimensional view of the shoulder as well as the ability to digitally rotate through the complete range of motion in any direction in order to choose the best possible implant for patients.
“We have committed ourselves to the development of our BLUEPRINT software platform that will deliver not only surgical planning but the creation of a digital ecosystem that has value elements for all of the stakeholders involved in patient care,” Dewey noted. “We believe that software-enabled solutions like BLUEPRINT are the future.”
Many of Wright Medical’s rivals share that philosophy as well: Exactech Inc. has devised software that instantly reconstructs the scapula in 3D, and Zimmer Biomet Holdings Inc. sells a preoperative planning platform that creates “pinpoint” accurate patient-specific surgical guides for anatomic and reverse shoulder arthroplasty candidates.
Both techniques are the focus of a Web platform developed by DePuy Synthes and Materialise NV to improve the digital planning, design, and ordering of patient-specific 3D printed guides for shoulder surgery. The platform debuted last spring at the American Academy of Orthopaedic Surgeons annual meeting.
“...this collaboration will empower more orthopaedic surgeons to discover the benefits of online planning...” Materialise founder/CEO Wilfried Vancraen declared at the time of the launch. “3D planning is gaining popularity with orthopedic surgeons performing shoulder operations.”
And that popularity is bound to grow over the next half-decade as more geriatrics seek to avoid infirmity and more athletes look to bypass (or briefly visit) injured lists.
ResearchAndMarkets.com data estimate the worldwide shoulder replacement market to grow 7.6 percent annually over the next five years to reach $1.9 billion. Potential headwinds during the forecast period include postoperative complications and implant costs.
“Patient needs will drive the development of new devices. As the market share grows for shoulder arthroplasty, revisions will become more frequent due to volume and the active aging population,” said Baptiste Martin, CEO of FX Shoulder USA Inc., the Dallas, Texas-based subsidiary of French firm FX Solutions S.A.S., that provides and distributes shoulder replacement devices in the United States. “There is a trend to follow revisions, particularly in cases involving bone loss of the glenoid, and to develop specific prostheses to treat patients and (potentially) improve outcomes and post-operative range of motion.”
FX Shoulder is focusing on the arthroplasty trend with its FDA-cleared Humeris and Humelock product portfolios; the Humelock II and Humelock Reversed are offered with optional screws (depending on surgeons’ preference for cement). FX Solutions also offers a stemless device (Easytech) for patients with arthritis and good bone quality, but it is now only approved in Europe.
The company, however, is attempting to expand its use across the pond, having launched in December 2018 an FDA-approved Investigational Device Exemption clinical study of the Easytech Reversed stemless shoulder prosthesis. Twelve U.S. surgeons at nine sites are participating in the trial.
“The Easytech Reversed may be exactly what the U.S. market is in need of,” Martin said, “and a reversed stemless prosthesis could potentially be the future of shoulder arthroplasty.”
It certainly seems that way. Market research shows a growing use of stemless implants in shoulder arthroplasty as surgeons seek to simplify revision procedures and prevent stem-related complications. Doctors also are finding an incentive for use through the increasing number of clinical studies proving the efficacy of stemless implants. A September 2017 study demonstrated nine-year clinical outcome comparability of stemless shoulder devices to that of third- and fourth-generation standard shoulder arthroplasty, as well as significant improvements in patient satisfaction.
Stemless implants are superficially similar to a resurfacing except for their thicker metaphyseal anchorage and larger component head. Contrary to their name, these devices have a short stem, but it does not enter the diaphysis of the bone like a stemmed implant; rather, it remains completely in the humeral neck mataphysis.
Despite its growing popularity, stemless arthroplasty is more prominent in Europe than the United States. Only a handful of these solutions are currently available in America, including Arthrex’s Eclipse, Wright Medical’s Simpliciti, Zimmer Biomet’s Sidus Stem-Free Shoulder, and Arthrosurface’s OVO Primary Stemless Shoulder System with Inlay Glenoid.
Arthrosurface’s OVO device was approved by the FDA last spring and first implanted in August. Touted as the only ovoid (egg)-shaped implant on the market, the OVO helps surgeons avoid overstuffing the joint, improves patients’ range of motion, and reduces pain. Moreover, the prosthesis uses a taper post that secures into dense, subchondral bone for strong fixation, and the inlay glenoid implant significantly reduces glenoid loosening due to the elimination of “rocking horse” effect.
“The shoulder market is exciting in the sheer volume of procedures, and the continued adoption and improvement of high-performance shoulder arthroplasty devices,” Arthrosurface CEO Steve Ek said. “The market now has many examples of devices that are entering the second and third generations of their designs. Therefore, I think we are starting to see more consistent and reproducible results with these devices. At the same time, patients and surgeons are starting to demand a greater level of performance and function from these devices. Where once the goal was just to mitigate pain, now I believe the expectation is the presumed elimination of pain but also the restoration of a high level of function.”
Care to verify that, Greg Bird?