Sean Fenske, Editor-in-Chief12.02.19
While every issue of ODT (and MPO for that matter) is significant (in my mind anyway), this one holds a little extra value because it’s the last editorial issue of the year for Mike, Sam, and I. After this, we look forward to our schedules winding down a bit throughout the holidays. This time of year, however, does give us a fantastic opportunity to get organized and “attack” the editorial schedule for the next year. That is, we’re provided a little more time to schedule contributed articles, contact potential columnists, and map out the responsibilities outlined in the editorial calendar. (That said, we’re always seeking new contributors and columnists; let me know if you’re interested!)
With this in mind, I thought I’d take a high-level view of some of the items the industry may want to consider getting a jump on for the coming year—“to do” list entries that haven’t yet been crossed off. What pending agenda still needs to be resolved within your organization? Following are a few to consider.
Device Tax: Sure, this first item may be resolved by the end of the year—or at least suspended for another year or two—but it should remain top of mind unless a full repeal happens. While it will take a legislative action to resolve this issue, it doesn’t mean your hands are tied in terms of attempting to bring attention to the issue. If you’re a member of MDMA, AdvaMed, MITA, or some other industry organization, you’ve no doubt heard about the continuing efforts to gain full repeal of this tax. As an individual within an industry harmed by this looming tax, you’re able to reach out to your representatives in Congress to call for action. Don’t sit back and hope others do the work on your behalf. Get involved, and at the very least, become familiar with the representatives for your region and ensure they’re aware of the reasons why this tax is so poor for the industry.
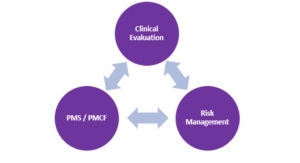
MDR: New regulatory changes are coming to Europe. Is your company currently selling products there? Are they ready to completely review every single SKU they have available in the EU? Are your customers (e.g., physicians, hospitals, healthcare facilities, etc.) aware of products you will no longer offer them due to corporate decisions not to have certain devices reevaluated under the new regulatory guidelines? Do you have product submissions coming up that may not make it under the May 2020 deadline and will need to comply with MDR? Is your Notified Body certified for MDR? Is your Notified Body even electing to be certified for MDR or have they dropped out? Is your Notified Body based in the U.K. and will therefore not be able to be certified to MDR because of Brexit? If you’re uncertain about any of these questions, you and/or someone at your company has work to do.
MDR stands to potentially disrupt healthcare in the EU significantly. While some are predicting delays, others expect device shortages that will directly impact the delivery of care. Sure, the deadline could be altered as it has been with Eudamed, but I don’t recommend hoping for that to occur as a business strategy. Find out what needs to be done to continue selling products in the EU or lose market share.
Bundled Payments: Payors have been introducing a new payment model referred to as bundled payments, where a complete procedure is provided reimbursement at a certain level. It’s then on the healthcare institution to deliver the required treatment as effectively and efficiently as possible. This includes the actual procedure, as well as any pre- and post-care. As a result, infection control, patient compliance, duration of stay, follow-up, and rehabilitation become key factors to consider and improve. If a healthcare facility can reduce infection rates, for example, they will retain a larger percentage of the bundled payment. As a result, care providers are seeking assistance from medical technology manufacturers to aid in this effort. Device makers that can offer a better degree of care (i.e., more efficient at a cheaper cost) through their technology offering will represent the cream that rises to the top. This presents a substantial financial opportunity for orthopedic device manufacturers. Be sure you’re making the case for your company’s technologies with healthcare organizations seeking help with this newer model.
Value-Based Healthcare: Bundled payments roll right into value-based healthcare—payment for outcomes over payment for service. The care delivery model is changing such that hospitals and healthcare organizations are being evaluated and reimbursed based on outcomes. The days of fee-for-service are coming to an end. Does your company have a story to tell that aids doctors in this effort? They are seeking your help; be sure you have the right answers for them. Otherwise, they will most certainly find help elsewhere.
Enjoy the upcoming holidays and hopefully some time off. Then return having already gotten a jump on the new year.
Sean Fenske, Editor-in-Chief
sfenske@rodmanmedia.com
With this in mind, I thought I’d take a high-level view of some of the items the industry may want to consider getting a jump on for the coming year—“to do” list entries that haven’t yet been crossed off. What pending agenda still needs to be resolved within your organization? Following are a few to consider.
Device Tax: Sure, this first item may be resolved by the end of the year—or at least suspended for another year or two—but it should remain top of mind unless a full repeal happens. While it will take a legislative action to resolve this issue, it doesn’t mean your hands are tied in terms of attempting to bring attention to the issue. If you’re a member of MDMA, AdvaMed, MITA, or some other industry organization, you’ve no doubt heard about the continuing efforts to gain full repeal of this tax. As an individual within an industry harmed by this looming tax, you’re able to reach out to your representatives in Congress to call for action. Don’t sit back and hope others do the work on your behalf. Get involved, and at the very least, become familiar with the representatives for your region and ensure they’re aware of the reasons why this tax is so poor for the industry.
MDR: New regulatory changes are coming to Europe. Is your company currently selling products there? Are they ready to completely review every single SKU they have available in the EU? Are your customers (e.g., physicians, hospitals, healthcare facilities, etc.) aware of products you will no longer offer them due to corporate decisions not to have certain devices reevaluated under the new regulatory guidelines? Do you have product submissions coming up that may not make it under the May 2020 deadline and will need to comply with MDR? Is your Notified Body certified for MDR? Is your Notified Body even electing to be certified for MDR or have they dropped out? Is your Notified Body based in the U.K. and will therefore not be able to be certified to MDR because of Brexit? If you’re uncertain about any of these questions, you and/or someone at your company has work to do.
MDR stands to potentially disrupt healthcare in the EU significantly. While some are predicting delays, others expect device shortages that will directly impact the delivery of care. Sure, the deadline could be altered as it has been with Eudamed, but I don’t recommend hoping for that to occur as a business strategy. Find out what needs to be done to continue selling products in the EU or lose market share.
Bundled Payments: Payors have been introducing a new payment model referred to as bundled payments, where a complete procedure is provided reimbursement at a certain level. It’s then on the healthcare institution to deliver the required treatment as effectively and efficiently as possible. This includes the actual procedure, as well as any pre- and post-care. As a result, infection control, patient compliance, duration of stay, follow-up, and rehabilitation become key factors to consider and improve. If a healthcare facility can reduce infection rates, for example, they will retain a larger percentage of the bundled payment. As a result, care providers are seeking assistance from medical technology manufacturers to aid in this effort. Device makers that can offer a better degree of care (i.e., more efficient at a cheaper cost) through their technology offering will represent the cream that rises to the top. This presents a substantial financial opportunity for orthopedic device manufacturers. Be sure you’re making the case for your company’s technologies with healthcare organizations seeking help with this newer model.
Value-Based Healthcare: Bundled payments roll right into value-based healthcare—payment for outcomes over payment for service. The care delivery model is changing such that hospitals and healthcare organizations are being evaluated and reimbursed based on outcomes. The days of fee-for-service are coming to an end. Does your company have a story to tell that aids doctors in this effort? They are seeking your help; be sure you have the right answers for them. Otherwise, they will most certainly find help elsewhere.
Enjoy the upcoming holidays and hopefully some time off. Then return having already gotten a jump on the new year.
Sean Fenske, Editor-in-Chief
sfenske@rodmanmedia.com