Cindy Boyer, Senior Manager, International Regulatory Affairs, MCRA LLC11.18.20
The EU Medical Device Regulation (MDR) is officially known as Regulation (EU) 2017/745 of the European Parliament and of the Council of April 5, 2017.1 It amends Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009. It repeals Council Directives 90/385/EEC and 93/42/EEC. MDR consolidates two existing legal provisions and replaces both the current Medical Device Directive (93/42/EEC) and the Active Implantable Medical Device Directive (90/385/EEC).2
Medtech companies must adopt the MDR for continued EU market access. Although the coronavirus pandemic prompted regulators to extend the deadline for EU MDR adoption by one year (now May 26, 2021), medical device manufacturers must be proactive and begin preparing now.
As an adjunct to the actual regulation, MDCG 2020-5: Clinical Evaluation – Equivalence. A guide for manufacturers and notified bodies (April 2020),3 was published and endorsed by the Medical Device Coordination Group (MDCG), a governing body comprised of EU Member States representatives and chaired by a delegate of the European Commission. However, the MDCG document is not a Commission document and does not reflect the agency’s official position. MDCG 2020-5’s authors acknowledge the guidance document is not legally binding, but rather should be regarded as “best practice.”3
This column focuses on the directive under EU 2017/745 on medical devices (MDR), highlighting some of the changes in claiming and demonstrating equivalence.
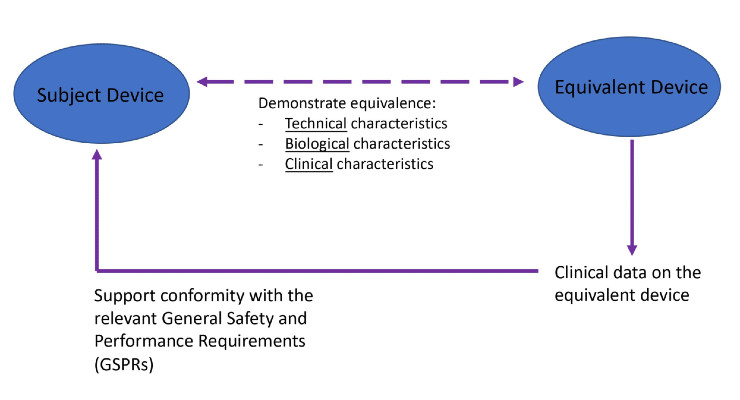
At a glance, to claim equivalence under the MDR:
Technical Characteristics: The MDR states devices are to be used under similar conditions of use, while MEDDEV 2.7/1 Rev. 4 requires devices be used under the same conditions of use1. “Conditions of use” encompasses environmental factors such as magnetic fields, temperature, moisture, and conditions during transport.
Interpretation: The conditions of use must be similar enough where there is no clinically significant difference in device safety and performance.
The MDR and MEDDEV 2.7/1 Rev. 4 have different examples/language describing similar specifications and properties. The listings are only examples and not exhaustive.
The MDR specifically states that software algorithms must be similar and the similarities should be based on the software algorithms’ functional principle, clinical performance, and intended purpose.
Equivalence does not have to be demonstrated for the software code. Software intended to for the configuration of a device (e.g. presentation on graphical user interface) and not related to a medicinal purpose (e.g. diagnostic, treatment, etc.) does not have to be similar.
Biological Characteristics
Under both the MDR and MEDDEV 2.7/1 Rev. 4, the subject and equivalent devices must use the same materials or substances. However, MEDDEV 2.7/1 Rev. 4 provides exceptions for devices that come in contact with intact skin, and minor device components.
Interpretation: The exception outlined in MEDDEV 2.7/1 Rev. 4 to not use the same materials is not acceptable under the MDR.
The MDR has additional text requiring similar release characteristics of substances to account for variability caused by processing, design, and use environment. For example, small changes in pH or oxidative stress can increase or decrease material release characteristics. It should be noted that properties and characteristics of the final device are to be considered.
Additional MDR requirements cover:
Clinical Characteristics
The MDR requires the kind of user to be the same. The regulation defines “user” as any healthcare professional or lay person who uses a device; a “lay person” is identified as an individual with no formal education in a relevant healthcare field or medicinal discipline.
Manufacturers must consider if the users are comparable. An example would be “professional use” vs. “home use.”
The MDR does not explicitly state the device has to be used for the same medical indication, gender, and duration of use (MEDDEV 2.7/1 Rev. 4 does). The MDR language is more general, but defines the end user through different language.
Demonstration of Equivalence
The MDR requires scientific justifications for claiming there are no clinically significant differences between a device’s technical, biological, and clinical characteristics. Each equivalent device must be independently equivalent to the subject device. Different parts of different devices cannot be used to claim equivalence to the subject device. One exception to this rule are “device systems” that involve several stand-alone products. In those cases, stand-alone devices within a system may claim equivalence to another stand-alone device within other systems, provided the system devices do not affect the safety and performance of one another.
Clinical Investigations
Besides the demonstration of equivalence, the MDR has specific requirements to avoid conducting a clinical investigation. Implantable and Class III devices require a clinical investigation unless they have been designed by modifications.
The Clinical Investigation Requirement for products without an Intended Medical Purpose (MDR Annex XVI) requires applicants to perform a clinical investigation unless existing data from an analogous device is justified. Analogous devices have a similar function and risk profile and are designed for a specific medical purpose. A clinical investigation would be needed for an equivalent product to a subject device with an aesthetic/non-medical purpose.
In summary, manufacturers must be cautious when claiming and demonstrating equivalence under the MDR. They need to specify and justify the level of clinical evidence necessary to demonstrate conformity with the relevant GSPRs, identify all clinical data (favorable and unfavorable) for both the subject and equivalent device. Additionally, differences between the subject and equivalent device must be discussed.
Similarly, manufacturers should avoid claiming equivalence if they cannot demonstrate sufficient levels of access to equivalent device data, and make claims to products without an intended medical purpose (listed in the MDR Annex XVI). Additionally, equivalence claims cannot be made for an ancillary medicinal substance as a “stand-alone” medicinal substance, or interchange claims to a non-ancillary medicinal substance if the subject device has an ancillary medicinal substance (and vice versa).
References
Cindy Boyer is an accomplished healthcare professional with over 15 years in the spine and cardiovascular medical device research and development expertise. At MCRA, Cindy supports international regulatory strategy and submissions for clientele. She contributes to the development of technical and regulatory documents. Using her biological and healthcare background to assist with pre-clinical and clinical strategies, she effectively communicates these strategies to internal team members and clients.
Medtech companies must adopt the MDR for continued EU market access. Although the coronavirus pandemic prompted regulators to extend the deadline for EU MDR adoption by one year (now May 26, 2021), medical device manufacturers must be proactive and begin preparing now.
As an adjunct to the actual regulation, MDCG 2020-5: Clinical Evaluation – Equivalence. A guide for manufacturers and notified bodies (April 2020),3 was published and endorsed by the Medical Device Coordination Group (MDCG), a governing body comprised of EU Member States representatives and chaired by a delegate of the European Commission. However, the MDCG document is not a Commission document and does not reflect the agency’s official position. MDCG 2020-5’s authors acknowledge the guidance document is not legally binding, but rather should be regarded as “best practice.”3
This column focuses on the directive under EU 2017/745 on medical devices (MDR), highlighting some of the changes in claiming and demonstrating equivalence.
At a glance, to claim equivalence under the MDR:
- Implants and Class III devices must include an agreement to access technical documentation for the equivalent device. (If the device is made by a different manufacturer, this type of access to proprietary information will, most likely, not be available).
- The technical criteria chosen to demonstrate equivalence must support the equivalence argument and critical performance specifications for the device under consideration. This should include product-specific standards or side-by-side testing of the equivalent device
- To show equivalency to an existing CE marked product, a table must be created that compares the supporting technical, biological, and clinical characteristics of the two devices. The Notified Body will question any differences and their significance to safety and performance. For significant differences, a medical professional needs to provide formal justification.
- Equivalency claims for products being initially CE Marked or sold in the EU need a solid justification to the Notified Body of how the data applies to an EU population This includes a thorough analysis of any safety and performance gaps related to the clinical performance.
Technical Characteristics: The MDR states devices are to be used under similar conditions of use, while MEDDEV 2.7/1 Rev. 4 requires devices be used under the same conditions of use1. “Conditions of use” encompasses environmental factors such as magnetic fields, temperature, moisture, and conditions during transport.
Interpretation: The conditions of use must be similar enough where there is no clinically significant difference in device safety and performance.
The MDR and MEDDEV 2.7/1 Rev. 4 have different examples/language describing similar specifications and properties. The listings are only examples and not exhaustive.
The MDR specifically states that software algorithms must be similar and the similarities should be based on the software algorithms’ functional principle, clinical performance, and intended purpose.
Equivalence does not have to be demonstrated for the software code. Software intended to for the configuration of a device (e.g. presentation on graphical user interface) and not related to a medicinal purpose (e.g. diagnostic, treatment, etc.) does not have to be similar.
Biological Characteristics
Under both the MDR and MEDDEV 2.7/1 Rev. 4, the subject and equivalent devices must use the same materials or substances. However, MEDDEV 2.7/1 Rev. 4 provides exceptions for devices that come in contact with intact skin, and minor device components.
Interpretation: The exception outlined in MEDDEV 2.7/1 Rev. 4 to not use the same materials is not acceptable under the MDR.
The MDR has additional text requiring similar release characteristics of substances to account for variability caused by processing, design, and use environment. For example, small changes in pH or oxidative stress can increase or decrease material release characteristics. It should be noted that properties and characteristics of the final device are to be considered.
Additional MDR requirements cover:
- Products composed of substances absorbed or locally dispersed in the body. Under Directive 2001/83/EC, the Notified Body must seek a scientific opinion from a competent authority or the European Medicines Agency (EMA)
- Devices that include an ancillary medicinal substance (e.g., a drug-eluting stent). The medicinal substance, and any excipients/coatings, must be the same substance. The Notified Body is required to verify the usefulness of the substance and seek a scientific opinion from a competent authority or EMA.
Clinical Characteristics
The MDR requires the kind of user to be the same. The regulation defines “user” as any healthcare professional or lay person who uses a device; a “lay person” is identified as an individual with no formal education in a relevant healthcare field or medicinal discipline.
Manufacturers must consider if the users are comparable. An example would be “professional use” vs. “home use.”
The MDR does not explicitly state the device has to be used for the same medical indication, gender, and duration of use (MEDDEV 2.7/1 Rev. 4 does). The MDR language is more general, but defines the end user through different language.
Demonstration of Equivalence
The MDR requires scientific justifications for claiming there are no clinically significant differences between a device’s technical, biological, and clinical characteristics. Each equivalent device must be independently equivalent to the subject device. Different parts of different devices cannot be used to claim equivalence to the subject device. One exception to this rule are “device systems” that involve several stand-alone products. In those cases, stand-alone devices within a system may claim equivalence to another stand-alone device within other systems, provided the system devices do not affect the safety and performance of one another.
Clinical Investigations
Besides the demonstration of equivalence, the MDR has specific requirements to avoid conducting a clinical investigation. Implantable and Class III devices require a clinical investigation unless they have been designed by modifications.
- Same manufacturer: The equivalent device must already be CE marked under the MDR or Directives 93/42/EEC or 90/385/EEC. The CE mark has to be current, and the Clinical Evaluation Report (CER) must be up-to-date with a favorable risk-benefit profile.
- External manufacturer: There must be a contract in place allowing full access to technical documentation on an ongoing basis. The original CER of the equivalent device must be MDR-compliant (not certified under Directives 93/42/EEC or 90/385/EEC). A manufacturer can claim equivalence to a device certified with respect to the MDR or Directives 93/42/EEC or 90/385/EEC.
- The manufacturer has sufficient access to data
- The equivalent device from an external manufacturer does not require contact between manufacturers for access to technical documentation
- Clinical investigations were conducted according to international guidelines
- Clinical data meets MDR requirements
The Clinical Investigation Requirement for products without an Intended Medical Purpose (MDR Annex XVI) requires applicants to perform a clinical investigation unless existing data from an analogous device is justified. Analogous devices have a similar function and risk profile and are designed for a specific medical purpose. A clinical investigation would be needed for an equivalent product to a subject device with an aesthetic/non-medical purpose.
In summary, manufacturers must be cautious when claiming and demonstrating equivalence under the MDR. They need to specify and justify the level of clinical evidence necessary to demonstrate conformity with the relevant GSPRs, identify all clinical data (favorable and unfavorable) for both the subject and equivalent device. Additionally, differences between the subject and equivalent device must be discussed.
Similarly, manufacturers should avoid claiming equivalence if they cannot demonstrate sufficient levels of access to equivalent device data, and make claims to products without an intended medical purpose (listed in the MDR Annex XVI). Additionally, equivalence claims cannot be made for an ancillary medicinal substance as a “stand-alone” medicinal substance, or interchange claims to a non-ancillary medicinal substance if the subject device has an ancillary medicinal substance (and vice versa).
References
Cindy Boyer is an accomplished healthcare professional with over 15 years in the spine and cardiovascular medical device research and development expertise. At MCRA, Cindy supports international regulatory strategy and submissions for clientele. She contributes to the development of technical and regulatory documents. Using her biological and healthcare background to assist with pre-clinical and clinical strategies, she effectively communicates these strategies to internal team members and clients.