Sam Brusco, Associate Editor11.18.20
Privately-held medical device company Embody Inc., which develops collagen-based technologies for sports medicine and soft tissue repair, gained FDA clearance for its TAPESTRY Biointengrative Implant for tendon and ligament repair just a few weeks ago. The bioengineered collagen-based implant with proprietary micro-architecture is a significant advancement in collagen science and could be a compelling solution with broad clinical applications for tendon repair. Thanks to initial funding from DARPA, Embody has pioneered additive manufacturing and advanced biofabrication to assemble collagen from the molecular scale. According to Smarttrak Biomed GPS, in 2019 over 2.4 million surgeries were performed in the U.S. to repair soft tissue injuries.
“There is clearly a clinical need to further improve upon our current healing rates in the treatment of many tendon and ligament injuries,” Kevin F. Bonner, M.D., an orthopedic surgeon and Director of the Research Foundation at The Jordan-Young Institute in Virginia Beach, Va., told the press. “Embody’s TAPESTRY implant represents a breakthrough in implant design that combines the benefits of collagen with the structural integrity required for demanding tendon and ligament applications from foot & ankle to complex shoulder repair procedures.”
Moving back almost 15 years, global medical device manufacturer Smith+Nephew released the TRIGEN INTERTAN Intertrochanteric Hip Fracture nail in July of 2006 for femoral fracture treatment. TRIGEN INTERTAN contains the company’s proprietary Integrated Compression Screws to provide a second point of fixation in the femoral head. These screws allow for mechanical compression through the implant, which is maintained after removing the instrument. The combination creates strong interfragmentary friction and boosts construct stability to resist rotation and varus collapse.
“Meta-analysis of data indicates the implant, which uses a proprietary Integrated Compression Screw, outperformed other single-screw intertrochanteric nails,” said John Clausen, vice president of global marketing, Trauma & Extremities, at Smith+Nephew. “Results show INTERTAN significantly reduced risk of implant related failures by 81 percent and revision by 65 percent vs. single-screw nails. The clinical evidence supporting its efficacy is overwhelming. Better patient outcomes mean better outcomes for hospitals, and we are thrilled to pass along these savings to the U.S. healthcare system.”
Smith+Nephew launched the INTERTAN Product Assurance (IPA) Program at last year’s Orthopaedic Trauma Association Annual Meeting. The program hopes to mitigate expenses in the hip fracture market by lowering the cost of repeat operations.
“Under the program, S+N will refund the cost of the implant to the facility for 12 months following the procedure if it fails to perform as expected,” explained Clausen. “With an estimated $12-15 billion annual cost and average 6.6 percent reoperation rate, hip fractures are among the most expensive fractures to care for in the healthcare system. The IPA Program is intended to help mitigate this expense by potentially reducing the overall reoperation rate.”
Periprosthetic fractures are fractures associated with an orthopedic implant, either a replacement or internal fixation device. Worldwide incidence of these fractures is increasing constantly due to the growing number of primary joint arthroplasties and revision surgeries. Further, patients demanding arthroplasty are becoming older and more active due to healthcare improvements.
“Periprosthetic fractures are on the rise,” said Clausen. “These cases are being treated by both trauma and reconstruction surgeons. Philosophies can widely differ between treatment protocols, making it difficult for companies to provide solutions acceptable for multiple surgeon types.”
The company launched the EVOS MINI Plating System for complex long bone fractures in 2014. It built on the growing trend of traumatologists reducing long bone fractures using a mini fragment system designed for the hand or foot. The EVOS MINI Plating System features three different, color-coded, size modules offering up to eight low-profile plate geometries.
“We have been focusing on our new EVOS Plating System and most recently in the area of large fragment and periprosthetic fractures,” said Clausen. “EVOS takes an evolutionary approach to simplifying and unifying plating systems by providing stability where needed and flexibility when required. EVOS is an expansive, user-friendly system with multiple fixation options including non-locking, locking, variable-angle locking, optimized plate contours, and screw trajectories as well as a low-profile construct.”
The EVOS WRIST Plating System launched at last September’s American Society for Surgery of the Hand Annual Meeting. It offers the range of approach, material, and locking technology needed to perform simple and complex wrist fracture surgeries in the way the surgeon chooses. EVOS WRIST touts both stainless steel and titanium volar plate options to encompass both variable-angle and fixed-angle locking technologies.
Patients needing deformity correction are among the most challenging cases orthopedic surgeons face. Over the last 25 years, according to Smith+Nephew, the company’s TAYLOR SPATIAL FRAME (TSF) external fixator has become the gold standard to treat complex limb deformity. The company is rolling out an enhancement to its TSF platform called TSF ALLY.
“Our TSF ALLY service supports surgeons with analysis and planning of patients selected for TSF surgery,” said Clausen. “Engineers trained in deformity correction use TraumaCad to analyze the deformity, create a TSF plan, and generate a case in spatialframe.com. TSF ALLY+ takes the support one step further by facilitating peer-to-peer consultation for the treating surgeon with an expert TSF surgeon, allowing clinical collaboration on a specific case. Through the collaboration of surgeon and expert engineer, TSF ALLY serves to optimize TSF treatment for the surgeon and patient by using dedicated software to conduct the X-ray analysis and accurately plan the hardware parameters.”
San Diego-based global orthopedic and spine technology manufacturer NuVasive Inc. is best known for its suite of solutions for less invasive spine surgery. However, the firm has a strong trauma and fixation offering as well.
“Currently in the trauma market, bone defect management solutions are met with high complication rates1, high infection rates, poor patient satisfaction, and significant time for a patient in an external fixation frame,” 2 said Chris Yunker, senior director of global product Management at NuVasive Specialized Orthopedics. “Large segmental defects of long bones are always a challenge in reconstructive surgery.”
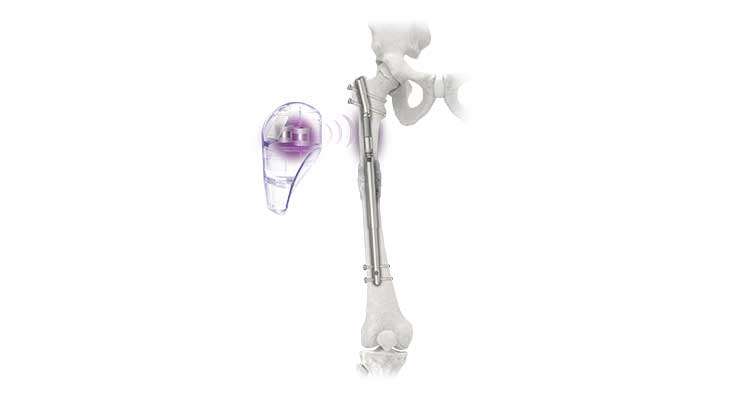
NuVasive’s Precice system is an intramedullary device introduced in 2011 that, once implanted, uses an External Remote Controller to non-invasively lengthen the femur and tibia. Through proprietary magnetic technology, each patient’s lengthening is customized based on the physician’s lengthening protocol. Customized programming allows lengthening sessions to be performed in the comfort of the patient’s own home. The less invasive procedure stimulates and regenerates bone growth without cumbersome external fixation. The third-generation limb-lengthening device—Precice Stryde—was used in its first procedures in May 2018. It features 400 percent increased post-op weight bearing compared to the original Precice—150-250 pounds vs. 30-50 pounds of weight on each leg. According to the company, earlier weight bearing may lead to quicker healing times for patients using the Precice System.
FDA clearance and CE mark approval was achieved for the Precice Bone Transport System last February. The implantable, magnetic intramedullary nail contains a dual slot to support transport of an intercalary bone segment to spur healthy regeneration to treat acute bone defects up to 10 centimeters. After it’s implanted, an external remote controller is used to move the bone segment up to 10 centimeters based on each patient’s need.
“In partnership with a limb reconstruction surgeon team, NSO has brought to market the Plate Assisted Bone Segment Transport (PABST) procedure, which includes the use of either the Precice Limb Lengthening device or the Precice Stryde Limb Lengthening device in conjunction with a long trauma plate, as well as the new Precice Bone Transport system, an all-internal, less invasive bone transport nail,” said Yunker.
The PABST procedure, released in 2018, can treat post-traumatic bone defects without limb shortening or an external fixator. It can potentially decrease post-op complications in bone defect reconstructive cases using all-internal technology designed for limb lengthening. It uses a magnetic intramedullary nail controlled with a handheld external remote allowing for precise, adjustable, and bidirectional bone segment transport.
“For decades, there has been limited innovation in the trauma fixation market, with competing companies having similar intramedullary nail, plate, and external fixation platforms with minimal differentiation,” said Yunker. “Surgeons demand improved and more gentle solutions for their patients and we see enabling instrumentation, software, and all-internal devices becoming the preferred standard of care.”
“NSO has embraced this change and is laser focused on development of disruptive technologies that address some of the most challenging clinical problems, while at the same time simplifying treatment options with enabling technologies,” Yunker continued. “With a focus on this complex reconstruction portion of the trauma market, NSO continues to displace the external fixator in limb lengthening cases with a remote-controlled compression treatment option for non-unions, and most recently an all-internal bone segment transport option that enables surgeon to treat a wide range of segmental bone defects caused by tumor or trauma.”
Reno, Nev.-based The Orthopaedic Implant Company (OIC) believes the way orthopedic implants are priced and supplied is flawed, to the detriment of everyone. The company intends to rethink and change that system by lowering the cost of orthopedic procedures, through development of more affordable implants with a balance of quality, service, and price for surgeons, hospitals, and patients. The ultimate goal is for OIC to play a role in creating a more fair and equitable healthcare environment through its view of the way healthcare items should be sourced and implants developed.
“Why are there often significant price disparities between implant manufacturers?” asked Itai Nemovicher, CEO and co-founder of OIC. “This is the real crux of our technology differentiation—it has nothing to do with the physical devices themselves, and everything to do with how we price and deliver those devices. Plenty of research supports the clinical equivalency of using our implants versus using implants from a big box manufacturer. Patients experience the same high-quality clinical outcomes, regardless of the implant being used. As a result, the financial component becomes the subsequent focal point. On average, hospitals that use our implants can realize a 56 percent reduction in costs3 compared to using traditional vendors’ pricing and delivery methods. In essence, hospitals, physicians, and patients get the benefit of high-quality outcomes while preventing a substantial amount of financial stress.”
It’s very important to have a value-based platform in today’s healthcare environment—it has the potential to work for everyone. Hospital operate on razor-thin margins or rely on taxpayer relief, sometimes unable to purchase and provide innovative treatments and technologies. Surgeons become overwhelmed with trying to squeeze more procedures into the same timeframes, encouraged to focus on numbers (i.e., minutes and margins) instead of patients. Patients are also plagued by healthcare costs that are upending lives after life-improving procedures.
“Our approach in the OR can help alleviate some these burdens,” said Nemovicher. “Sales rep presence in the OR is expensive and reflected in conventional implant vendors’ prices. While we recognize clinical representation has value and merit, it’s not necessary for every single case, so we work with hospitals and surgeons to provide the exact level of support needed without unnecessary, excessive costs. We also know routine and consistency for surgeons can save time and impact outcomes, so by virtue of necessity, our implants are designed to be comprehensive and incredibly intuitive so surgical teams can operate with ease and efficiency.”
OIC’s portfolio runs the gamut of trauma and fixation products. It includes cannulated screws; external fixation; hip and tibia intramedullary nails; a trochanteric antegrade nail; an antegrade/retrograde nail; plate and screw systems for the proximal tibia, proximal humerus, distal fibula, clavicle, distal radius, and distal tibia; and a 5.5 mm suture anchor for sports medicine applications.
“In a study conducted by the University of Nevada – Reno, use of our implants cut down OR time by 11 minutes on average,” said Nemovicher. “Lowering healthcare costs for patients is urgently important. Surgeons can provide a new standard of stewardship for their patients when using our products to do what’s best for them clinically and financially.”
The company celebrated its 10-year anniversary in October, and was awarded ISO 13485:2016 certification shortly after, designating that all of the company’s implants meet the most up-to-date regulatory requirements of the medical device industry. To receive certification, a company must undergo intense internal audits and pass a two-stage registrar audit conducted by an external party. Certification demonstrates to regulators that high quality standards have been met.
“This ISO certification confirms that value doesn’t mean quality is sacrificed. Our systems and implants are developed, manufactured and delivered in alignment with the highest quality standards but without the exorbitant price tag,” Todd Martens, vice president of product development of OIC, told the press.
“We partnered with Intermountain Healthcare, one of the 30 largest health systems in the country, in fall 2018 to help them examine the best ways to create value within their orthopedic practices,” said Nemovicher. “So far, the preliminary data when using our implants strongly coincides with what we’ve seen historically with other hospitals (between 45-55 percent overall savings) compared to conventional implant vendors.”
This pilot program has resulted in a $3.6 million reduction on supply expenses so far. Intermountain’s overall strategy intends to have more of a macroeceonomic impact, lowering insurance premiums for people in the community it serves. Intermountain leaders have credited the program’s success in part to surgeons who involved themselves in the process, assisting in making decisions and working with sales reps on discounts that complied with the program.
Metal implants have been the standard of care for trauma surgery, and this has remained fairly stagnant for over a century. Trauma or orthopedic surgery’s goal is to return the patient to a pre-injury state. Metal hardware can obstruct the body’s natural healing process and cause discomfort, pain, and temperature sensitivity. Sometimes, the patient must even undergo a secondary removal surgery.
“This has warranted the age-old question; how can we heal bones better?” said Brian Verrier, CEO of OSSIO, a Woburn-Mass.-based orthopedic fixation company committed to transforming the orthopedic experience for patients, physicians and payors. “OSSIOfiber Intelligent Bone Regeneration Technology enables surgeons to leverage existing surgical techniques honed over years with OSSIOfiber implants that provide the same necessary bone fixation strength as metal, but integrate into bone or tissue over time. The patient’s healthy bone is restored without any remaining permanent metal hardware.”
OSSIOfiber implants aim to reinforce and restore healthy bone, leaving the body renewed without metal or permanent hardware. The first-of-its-kind implant material securely fixates and integrates into native anatomy without anything left behind. OSSIOfiber contains the same organic minerals found in bones themselves, but the company claims it is stronger—it eventually becomes part of the bone. This encourages a return to full strength naturally without the risks and costs tied to permanent hardware.4
“Initially, we are focusing on internal fixation for extremities (i.e. foot/ankle, hand/wrist) while expanding our portfolio of bio-integrative bone fixation solutions to enter into other specialties such as sports medicine, pediatrics, and spine in the future,” said Verrier.
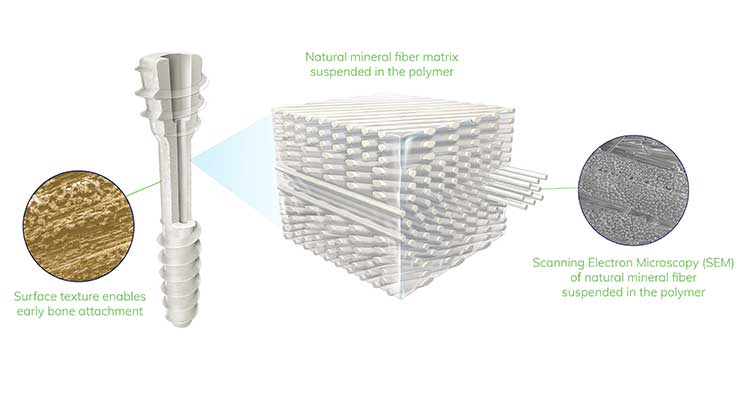
The OSSIOfiber Hammertoe Fixation System won FDA clearance this past March for maintenance of alignment and fixation of bone fractures, osteotomies, and bone grafts. The system utilizes the company’s Intelligent Bone Regeneration Technology, a proprietary natural mineral fiber matrix that is more biologically friendly in restoring patient stability and mobility. European multi-center clinical trial results from last year demonstrated fusion rates well above historical literature, improvements in pain and quality of life scores, radiographic evidence of good bio-integration with surrounding anatomy, and no evidence of serious adverse biological response or events.
The OSSIOfiber Trimmable Fixation Nail System was launched in the U.S. in July. Available in 2.4 mm and 4 mm diameters and packaged with sterile, disposable instrumentation, the trimmable nails rival performance of metal compression screws. Ability to trim the implants to any desired length allows for customization to any patient anatomy. The nails can also be adapted for forefoot, midfoot, hindfoot, and an array of hand/wrist applications. The nail’s design encourages maintenance of compression, stability, fixation strength, and strong pull-out strength. The nail’s hexagonal shape resist rotation as well, preventing loosening that often culminates in screw back-out.
Just a few weeks ago the OSSIOfiber Compression Screw Portfolio earned an FDA nod for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle, and foot in the presence of appropriate brace and/or immobilization. The portfolio will first comprise a 4 mm-diameter cannulated, headless, partially threaded compression screw that range in length from 26 to 60 mm. OSSIO plans to commercially launch the compression screws in the U.S. early next year and expand the portfolio in varying diameters, lengths, and geometry. The company also announced its 1,000th procedural milestone in October between the Hammertoe Fixation System and Trimmable Fixation Nail System.
“We plan to expand our trauma portfolio with additional strong, bio-integrative fixation solutions while leveraging existing surgical techniques, existing reimbursement, and our breakthrough implant material that is 1.5 stronger than cortical bone and fully integrates into surrounding anatomy without adverse inflammation—leaving patients renewed without permanent hardware,” said Verrier. “This includes a broader range of screws, cannulated fixation nails, staples, anchors, and plates.”
Elective surgeries being stifled by the COVID-19 pandemic have touched essentially all facets of the orthopedic industry. Accidents do still happen, though, so orthopedic trauma surgeries have not been nearly as affected. However, with increased volume of patients in the hospital as another wave of infection prepares to strike during the winter, it’s important that trauma and fixation technologies work in such a way to prevent secondary operations.
“COVID-19 hasn’t impacted trauma surgery the way elective surgeries have been,” said Verrier. “If anything, the desire for reliable solutions that can avoid the patient returning for a potential secondary operation due to hardware removal is more attractive than ever. Sterile, disposable instrumentation that complement OSSIOfiber implants also reduce OR preparation time, resources, and cross-patient contamination risk by not having to clean and re-sterilize large instrumentation trays.”
References
“There is clearly a clinical need to further improve upon our current healing rates in the treatment of many tendon and ligament injuries,” Kevin F. Bonner, M.D., an orthopedic surgeon and Director of the Research Foundation at The Jordan-Young Institute in Virginia Beach, Va., told the press. “Embody’s TAPESTRY implant represents a breakthrough in implant design that combines the benefits of collagen with the structural integrity required for demanding tendon and ligament applications from foot & ankle to complex shoulder repair procedures.”
Moving back almost 15 years, global medical device manufacturer Smith+Nephew released the TRIGEN INTERTAN Intertrochanteric Hip Fracture nail in July of 2006 for femoral fracture treatment. TRIGEN INTERTAN contains the company’s proprietary Integrated Compression Screws to provide a second point of fixation in the femoral head. These screws allow for mechanical compression through the implant, which is maintained after removing the instrument. The combination creates strong interfragmentary friction and boosts construct stability to resist rotation and varus collapse.
“Meta-analysis of data indicates the implant, which uses a proprietary Integrated Compression Screw, outperformed other single-screw intertrochanteric nails,” said John Clausen, vice president of global marketing, Trauma & Extremities, at Smith+Nephew. “Results show INTERTAN significantly reduced risk of implant related failures by 81 percent and revision by 65 percent vs. single-screw nails. The clinical evidence supporting its efficacy is overwhelming. Better patient outcomes mean better outcomes for hospitals, and we are thrilled to pass along these savings to the U.S. healthcare system.”
Smith+Nephew launched the INTERTAN Product Assurance (IPA) Program at last year’s Orthopaedic Trauma Association Annual Meeting. The program hopes to mitigate expenses in the hip fracture market by lowering the cost of repeat operations.
“Under the program, S+N will refund the cost of the implant to the facility for 12 months following the procedure if it fails to perform as expected,” explained Clausen. “With an estimated $12-15 billion annual cost and average 6.6 percent reoperation rate, hip fractures are among the most expensive fractures to care for in the healthcare system. The IPA Program is intended to help mitigate this expense by potentially reducing the overall reoperation rate.”
Periprosthetic fractures are fractures associated with an orthopedic implant, either a replacement or internal fixation device. Worldwide incidence of these fractures is increasing constantly due to the growing number of primary joint arthroplasties and revision surgeries. Further, patients demanding arthroplasty are becoming older and more active due to healthcare improvements.
“Periprosthetic fractures are on the rise,” said Clausen. “These cases are being treated by both trauma and reconstruction surgeons. Philosophies can widely differ between treatment protocols, making it difficult for companies to provide solutions acceptable for multiple surgeon types.”
The company launched the EVOS MINI Plating System for complex long bone fractures in 2014. It built on the growing trend of traumatologists reducing long bone fractures using a mini fragment system designed for the hand or foot. The EVOS MINI Plating System features three different, color-coded, size modules offering up to eight low-profile plate geometries.
“We have been focusing on our new EVOS Plating System and most recently in the area of large fragment and periprosthetic fractures,” said Clausen. “EVOS takes an evolutionary approach to simplifying and unifying plating systems by providing stability where needed and flexibility when required. EVOS is an expansive, user-friendly system with multiple fixation options including non-locking, locking, variable-angle locking, optimized plate contours, and screw trajectories as well as a low-profile construct.”
The EVOS WRIST Plating System launched at last September’s American Society for Surgery of the Hand Annual Meeting. It offers the range of approach, material, and locking technology needed to perform simple and complex wrist fracture surgeries in the way the surgeon chooses. EVOS WRIST touts both stainless steel and titanium volar plate options to encompass both variable-angle and fixed-angle locking technologies.
Patients needing deformity correction are among the most challenging cases orthopedic surgeons face. Over the last 25 years, according to Smith+Nephew, the company’s TAYLOR SPATIAL FRAME (TSF) external fixator has become the gold standard to treat complex limb deformity. The company is rolling out an enhancement to its TSF platform called TSF ALLY.
“Our TSF ALLY service supports surgeons with analysis and planning of patients selected for TSF surgery,” said Clausen. “Engineers trained in deformity correction use TraumaCad to analyze the deformity, create a TSF plan, and generate a case in spatialframe.com. TSF ALLY+ takes the support one step further by facilitating peer-to-peer consultation for the treating surgeon with an expert TSF surgeon, allowing clinical collaboration on a specific case. Through the collaboration of surgeon and expert engineer, TSF ALLY serves to optimize TSF treatment for the surgeon and patient by using dedicated software to conduct the X-ray analysis and accurately plan the hardware parameters.”
San Diego-based global orthopedic and spine technology manufacturer NuVasive Inc. is best known for its suite of solutions for less invasive spine surgery. However, the firm has a strong trauma and fixation offering as well.
“Currently in the trauma market, bone defect management solutions are met with high complication rates1, high infection rates, poor patient satisfaction, and significant time for a patient in an external fixation frame,” 2 said Chris Yunker, senior director of global product Management at NuVasive Specialized Orthopedics. “Large segmental defects of long bones are always a challenge in reconstructive surgery.”
NuVasive’s Precice system is an intramedullary device introduced in 2011 that, once implanted, uses an External Remote Controller to non-invasively lengthen the femur and tibia. Through proprietary magnetic technology, each patient’s lengthening is customized based on the physician’s lengthening protocol. Customized programming allows lengthening sessions to be performed in the comfort of the patient’s own home. The less invasive procedure stimulates and regenerates bone growth without cumbersome external fixation. The third-generation limb-lengthening device—Precice Stryde—was used in its first procedures in May 2018. It features 400 percent increased post-op weight bearing compared to the original Precice—150-250 pounds vs. 30-50 pounds of weight on each leg. According to the company, earlier weight bearing may lead to quicker healing times for patients using the Precice System.
FDA clearance and CE mark approval was achieved for the Precice Bone Transport System last February. The implantable, magnetic intramedullary nail contains a dual slot to support transport of an intercalary bone segment to spur healthy regeneration to treat acute bone defects up to 10 centimeters. After it’s implanted, an external remote controller is used to move the bone segment up to 10 centimeters based on each patient’s need.
“In partnership with a limb reconstruction surgeon team, NSO has brought to market the Plate Assisted Bone Segment Transport (PABST) procedure, which includes the use of either the Precice Limb Lengthening device or the Precice Stryde Limb Lengthening device in conjunction with a long trauma plate, as well as the new Precice Bone Transport system, an all-internal, less invasive bone transport nail,” said Yunker.
The PABST procedure, released in 2018, can treat post-traumatic bone defects without limb shortening or an external fixator. It can potentially decrease post-op complications in bone defect reconstructive cases using all-internal technology designed for limb lengthening. It uses a magnetic intramedullary nail controlled with a handheld external remote allowing for precise, adjustable, and bidirectional bone segment transport.
“For decades, there has been limited innovation in the trauma fixation market, with competing companies having similar intramedullary nail, plate, and external fixation platforms with minimal differentiation,” said Yunker. “Surgeons demand improved and more gentle solutions for their patients and we see enabling instrumentation, software, and all-internal devices becoming the preferred standard of care.”
“NSO has embraced this change and is laser focused on development of disruptive technologies that address some of the most challenging clinical problems, while at the same time simplifying treatment options with enabling technologies,” Yunker continued. “With a focus on this complex reconstruction portion of the trauma market, NSO continues to displace the external fixator in limb lengthening cases with a remote-controlled compression treatment option for non-unions, and most recently an all-internal bone segment transport option that enables surgeon to treat a wide range of segmental bone defects caused by tumor or trauma.”
Reno, Nev.-based The Orthopaedic Implant Company (OIC) believes the way orthopedic implants are priced and supplied is flawed, to the detriment of everyone. The company intends to rethink and change that system by lowering the cost of orthopedic procedures, through development of more affordable implants with a balance of quality, service, and price for surgeons, hospitals, and patients. The ultimate goal is for OIC to play a role in creating a more fair and equitable healthcare environment through its view of the way healthcare items should be sourced and implants developed.
“Why are there often significant price disparities between implant manufacturers?” asked Itai Nemovicher, CEO and co-founder of OIC. “This is the real crux of our technology differentiation—it has nothing to do with the physical devices themselves, and everything to do with how we price and deliver those devices. Plenty of research supports the clinical equivalency of using our implants versus using implants from a big box manufacturer. Patients experience the same high-quality clinical outcomes, regardless of the implant being used. As a result, the financial component becomes the subsequent focal point. On average, hospitals that use our implants can realize a 56 percent reduction in costs3 compared to using traditional vendors’ pricing and delivery methods. In essence, hospitals, physicians, and patients get the benefit of high-quality outcomes while preventing a substantial amount of financial stress.”
It’s very important to have a value-based platform in today’s healthcare environment—it has the potential to work for everyone. Hospital operate on razor-thin margins or rely on taxpayer relief, sometimes unable to purchase and provide innovative treatments and technologies. Surgeons become overwhelmed with trying to squeeze more procedures into the same timeframes, encouraged to focus on numbers (i.e., minutes and margins) instead of patients. Patients are also plagued by healthcare costs that are upending lives after life-improving procedures.
“Our approach in the OR can help alleviate some these burdens,” said Nemovicher. “Sales rep presence in the OR is expensive and reflected in conventional implant vendors’ prices. While we recognize clinical representation has value and merit, it’s not necessary for every single case, so we work with hospitals and surgeons to provide the exact level of support needed without unnecessary, excessive costs. We also know routine and consistency for surgeons can save time and impact outcomes, so by virtue of necessity, our implants are designed to be comprehensive and incredibly intuitive so surgical teams can operate with ease and efficiency.”
OIC’s portfolio runs the gamut of trauma and fixation products. It includes cannulated screws; external fixation; hip and tibia intramedullary nails; a trochanteric antegrade nail; an antegrade/retrograde nail; plate and screw systems for the proximal tibia, proximal humerus, distal fibula, clavicle, distal radius, and distal tibia; and a 5.5 mm suture anchor for sports medicine applications.
“In a study conducted by the University of Nevada – Reno, use of our implants cut down OR time by 11 minutes on average,” said Nemovicher. “Lowering healthcare costs for patients is urgently important. Surgeons can provide a new standard of stewardship for their patients when using our products to do what’s best for them clinically and financially.”
The company celebrated its 10-year anniversary in October, and was awarded ISO 13485:2016 certification shortly after, designating that all of the company’s implants meet the most up-to-date regulatory requirements of the medical device industry. To receive certification, a company must undergo intense internal audits and pass a two-stage registrar audit conducted by an external party. Certification demonstrates to regulators that high quality standards have been met.
“This ISO certification confirms that value doesn’t mean quality is sacrificed. Our systems and implants are developed, manufactured and delivered in alignment with the highest quality standards but without the exorbitant price tag,” Todd Martens, vice president of product development of OIC, told the press.
“We partnered with Intermountain Healthcare, one of the 30 largest health systems in the country, in fall 2018 to help them examine the best ways to create value within their orthopedic practices,” said Nemovicher. “So far, the preliminary data when using our implants strongly coincides with what we’ve seen historically with other hospitals (between 45-55 percent overall savings) compared to conventional implant vendors.”
This pilot program has resulted in a $3.6 million reduction on supply expenses so far. Intermountain’s overall strategy intends to have more of a macroeceonomic impact, lowering insurance premiums for people in the community it serves. Intermountain leaders have credited the program’s success in part to surgeons who involved themselves in the process, assisting in making decisions and working with sales reps on discounts that complied with the program.
Metal implants have been the standard of care for trauma surgery, and this has remained fairly stagnant for over a century. Trauma or orthopedic surgery’s goal is to return the patient to a pre-injury state. Metal hardware can obstruct the body’s natural healing process and cause discomfort, pain, and temperature sensitivity. Sometimes, the patient must even undergo a secondary removal surgery.
“This has warranted the age-old question; how can we heal bones better?” said Brian Verrier, CEO of OSSIO, a Woburn-Mass.-based orthopedic fixation company committed to transforming the orthopedic experience for patients, physicians and payors. “OSSIOfiber Intelligent Bone Regeneration Technology enables surgeons to leverage existing surgical techniques honed over years with OSSIOfiber implants that provide the same necessary bone fixation strength as metal, but integrate into bone or tissue over time. The patient’s healthy bone is restored without any remaining permanent metal hardware.”
OSSIOfiber implants aim to reinforce and restore healthy bone, leaving the body renewed without metal or permanent hardware. The first-of-its-kind implant material securely fixates and integrates into native anatomy without anything left behind. OSSIOfiber contains the same organic minerals found in bones themselves, but the company claims it is stronger—it eventually becomes part of the bone. This encourages a return to full strength naturally without the risks and costs tied to permanent hardware.4
“Initially, we are focusing on internal fixation for extremities (i.e. foot/ankle, hand/wrist) while expanding our portfolio of bio-integrative bone fixation solutions to enter into other specialties such as sports medicine, pediatrics, and spine in the future,” said Verrier.
The OSSIOfiber Hammertoe Fixation System won FDA clearance this past March for maintenance of alignment and fixation of bone fractures, osteotomies, and bone grafts. The system utilizes the company’s Intelligent Bone Regeneration Technology, a proprietary natural mineral fiber matrix that is more biologically friendly in restoring patient stability and mobility. European multi-center clinical trial results from last year demonstrated fusion rates well above historical literature, improvements in pain and quality of life scores, radiographic evidence of good bio-integration with surrounding anatomy, and no evidence of serious adverse biological response or events.
The OSSIOfiber Trimmable Fixation Nail System was launched in the U.S. in July. Available in 2.4 mm and 4 mm diameters and packaged with sterile, disposable instrumentation, the trimmable nails rival performance of metal compression screws. Ability to trim the implants to any desired length allows for customization to any patient anatomy. The nails can also be adapted for forefoot, midfoot, hindfoot, and an array of hand/wrist applications. The nail’s design encourages maintenance of compression, stability, fixation strength, and strong pull-out strength. The nail’s hexagonal shape resist rotation as well, preventing loosening that often culminates in screw back-out.
Just a few weeks ago the OSSIOfiber Compression Screw Portfolio earned an FDA nod for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle, and foot in the presence of appropriate brace and/or immobilization. The portfolio will first comprise a 4 mm-diameter cannulated, headless, partially threaded compression screw that range in length from 26 to 60 mm. OSSIO plans to commercially launch the compression screws in the U.S. early next year and expand the portfolio in varying diameters, lengths, and geometry. The company also announced its 1,000th procedural milestone in October between the Hammertoe Fixation System and Trimmable Fixation Nail System.
“We plan to expand our trauma portfolio with additional strong, bio-integrative fixation solutions while leveraging existing surgical techniques, existing reimbursement, and our breakthrough implant material that is 1.5 stronger than cortical bone and fully integrates into surrounding anatomy without adverse inflammation—leaving patients renewed without permanent hardware,” said Verrier. “This includes a broader range of screws, cannulated fixation nails, staples, anchors, and plates.”
Elective surgeries being stifled by the COVID-19 pandemic have touched essentially all facets of the orthopedic industry. Accidents do still happen, though, so orthopedic trauma surgeries have not been nearly as affected. However, with increased volume of patients in the hospital as another wave of infection prepares to strike during the winter, it’s important that trauma and fixation technologies work in such a way to prevent secondary operations.
“COVID-19 hasn’t impacted trauma surgery the way elective surgeries have been,” said Verrier. “If anything, the desire for reliable solutions that can avoid the patient returning for a potential secondary operation due to hardware removal is more attractive than ever. Sterile, disposable instrumentation that complement OSSIOfiber implants also reduce OR preparation time, resources, and cross-patient contamination risk by not having to clean and re-sterilize large instrumentation trays.”
|
The orthopedic trauma market stands on its own as one of the few comprised of non-elective surgeries. But firms manufacturing trauma/fixation technologies and components for orthopedic OEMs have the same demands for precision, speed, and quality as any other type of implantable medical device. To gain perspective on challenges and best practices for working with orthopedic trauma and fixation OEMs, ODT spoke with Greg Neher, engineering manager at Precision Medical Technologies, a Warsaw, Ind.-based contract manufacturer of orthopedic implants and instruments. “One of the challenges I have faced with OEMs is making process improvements. “It is a long process and can consume engineering resources to complete all the documentation and testing requirements the OEM needs to approve a supplier change request. It can force us to run product with less-than-ideal processes to meet demand while waiting for approvals.” “The second challenge I have seen is with transfer work of legacy product. With legacy product, there can be print or model issues resolved with previous suppliers through phone calls or email, but prints and/or models were not updated accordingly. When the work is transferred, the resources aren’t as available to work through the issues at the OEM as they would be on a new product launch.” “One of the best practices I have seen across the board is a combined pre-production meeting with our team and the OEM’s team once a project has been awarded. This first meeting is great for introductions to determine the best contact at the OEM as issues arise. The pre-production meeting also helps us clearly define the project’s deliverables and helps with the transfer of critical or tribal knowledge.” |
References
- Paley D, Maar DC. Ilizarov bone transport treatment for tibial defects. J Orthop Trauma 2000:14(2):76-85.
- Kadhim M, Holmes L, Gesheff, MG, et al. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma 2017;31(2):111-119.
- J Orthop Trauma, Volume 30, Number 12 Supplement, December 2016
- Data on File at OSSIO