Jeph Ruppert and Steve Ward, 3D Systems05.17.21
Additive manufacturing (AM) has revolutionized orthopedics by improving the performance of implant design and patient-matched solutions to treat cases previously thought untreatable. The use of AM for medical applications continues to expand as physicians, researchers, and medical device companies exploit the technology’s flexibility to develop new applications.
Yet with all the amazing innovation, those that are deeply engaged with medical device manufacturing on a daily basis are cognizant of the potential to improve a patient’s life, but also realize we operate in a highly regulated industry. As a result, not only does the device require validation and clearance, the process used to create the device must also be characterized and validated.
Over the past decade, direct metal printing (DMP)—also known as laser powder-bed fusion (LPBF)—has matured significantly and is widely embraced by medical device manufacturers to produce metal components with enhanced functionality and performance to improve clinical outcomes. DMP components for such devices are often manufactured in Ti6Al4V because of the titanium alloy's compelling material properties, such as biocompatibility, high-strength-to-weight ratio, and excellent fatigue properties, along with its extensive current use in the industry. Medical device design and manufacturing is subject to rigid validation/qualification procedures and quality assurance requirements. Hence, stable and capable manufacturing processes are required to drive adoption of the manufacturing technology and the products created from it.
Today, over 100 FDA clearances have been granted for medical devices produced via AM. To help manufacturers navigate the AM process, the FDA released a guidance document in December 2017. This document not only adds credibility to AM as a viable solution for medical device manufacturing, it also offers risk-based, process-driven considerations for the development and manufacture of medical devices using a wide range of AM technologies. It does not, however, act as a stipulative document, thus leaving some flexibility to the process owner for implementation and execution.
Anyone who has integrated AM into a traditional manufacturing workflow can tell you it’s a daunting process. When you integrate AM into a highly regulated medical device workflow, the degree of difficulty skyrockets. Many find the key to an efficient, successful integration of AM is to partner with an experienced organization. There is power in the knowledge and expertise that comes from not only selecting the right solution to address a specific application, but then designing and manufacturing the device, and completing the submission to the FDA.
For example, of the previously mentioned FDA clearances, 3D Systems was involved in a majority of the applications. To date, we’ve produced more than 2 million devices and supported over 100 CE-marked and FDA-cleared devices. A catalyst for this activity is the quality approach, validation strategy, and quality management system we employ, which is aligned to the FDA’s guidance document. The validation strategy can greatly reduce the amount of front-end testing required for a medical device submission, and working with an experienced supplier speeds the time to market and removes risk from the development process. We’ve been able to help manufacturers accelerate their timeline for new market introductions to as little as nine months from concept through FDA clearance.
For those medical device manufacturers looking to integrate DMP into their workflow, or tackle new applications with the technology, it’s important to address several components during process development and validation.
Process Controls
A critical assessment DMP qualification for medical device production relies on a risk-based analysis of the entire DMP process flow—from powder feedstock to printed part. Figure 1 offers a simplified flow chart covering these components.
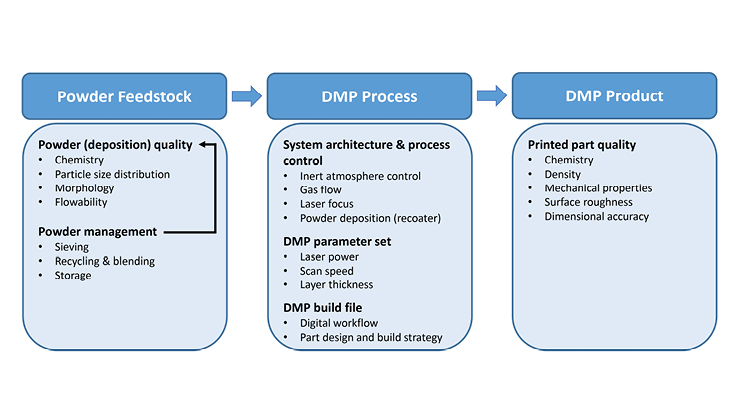
Figure 1: Simplified DMP process flow including (but not limited to) variables and characteristics of the powder feedstock, process characterization/validation, and the DMP product.
Let’s explore the components of this workflow in more detail.
Powder Feedstock
Quality assurance of the powder feedstock is the first prerequisite for obtaining high-quality and reproducible DMP products. The feedstock quality relies on the powder chemistry and intrinsic powder properties such as particle size distribution (PSD), powder morphology, and the associated flowability. The powder quality is affected by these properties combined with the powder surface chemistry (i.e., moisture and oxygen content). Ultimately, powder quality is a determining factor in the end-use properties of the device and is preeminently important to control adequately.
DMP Process
Exhibiting a stable DMP process is essential to pass the strict validation process, thereby delivering reliable DMP products with excellent reproducible part quality. DMP process stability can be affected by three main process variables: DMP machine condition, DMP parameter set, and/or DMP build file, as illustrated in Figure 1.
Robust DMP Machine Architecture
A stable DMP process with a low build-to-build and machine-to-machine variability starts with a robust machine architecture and appropriate machine calibration procedures. The DMP platform architecture that underlies the DMP Flex 350, DMP Factory 350, and DMP Factory 500 involves a vacuum pre-cycle prior to the printing job that actively removes air and moisture from the build chamber and the powder bed, and fills it afterward with high-purity argon gas. The event of extracting the oxygen and moisture from the powder feed is called the "boiling effect" and is shown in Figure 2.

Figure 2: Boiling effect showing the extraction of oxygen and moisture from the powder feed.1
The efficient/effective vacuum pre-cycle helps achieve extremely low oxygen in the build chamber. Furthermore, the DMP machine’s design ensures no oxygen can leak into the build chamber and, therefore, results in minimal argon consumption during printing. This vacuum chamber concept is key for the desired tight control of the inert atmosphere during printing. Additionally, the vacuum chamber concept helps to eliminate the risks for oxygen pick up by the powder feedstock, resulting in stable powder chemistry and a significant enhancement of the powder reusability of Ti Gr23.1
Poor control of the inert atmosphere (i.e., oxygen and moisture) during the build will lead to an oxygen increase in the printed material. This oxygen enrichment forms a substantial risk for the deterioration of the material’s properties. Therefore, tight control of the inert atmosphere in the build chamber is necessary for extended powder reusability and for obtaining excellent, repeatable mechanical part properties.
DMP Build File
Digital data exchange through software workflows is an essential element of the AM process. Securing digital data exchange and validating software workflows are part of the quality assurance requirements and the DMP qualification process. Developers should seek a software package for AM that enables the user to prepare, optimize, and 3D print high-quality parts—streamlining the workflow from design to post-processing. The software’s job file should contain all parameter data and dimensional data for the part, which can be translated across machines in the same state of maintenance at any location.
In conclusion, quality assurance and monitoring of powder feedstock, combined with a robust DMP process, well-documented machine calibration protocols, and trained machine operators all play a vital role in achieving reliable and repeatable print-to-print quality, yielding fully dense and excellent mechanical properties and surface roughness.
Process Characterization and Qualification
Now that we have all the components defined, we can turn our attention to how the process is characterized and qualified. This begins with the development of a Critical-to-Quality versus Critical Process Parameter (CTQ/CPP) matrix to determine influences and create a pathway to develop studies and establish objective evidence before moving into full process validation. The objective is to ensure all parameters are well-tested and validated while limiting time and incurred cost. The output of the CTQ/CPP matrix is used as input to a process characterization design of experiment and eventually flows into an operational qualification, providing challenge variables and a process window.
To demonstrate this, Figure 3 is the DMP qualification flowchart. The DMP qualification process relies on a risk-based approach and follows a phase-gated flow, passing through process validation, equipment validation, and product validation to achieve qualified product production. The DMP qualification process complies with ASTM F3434.
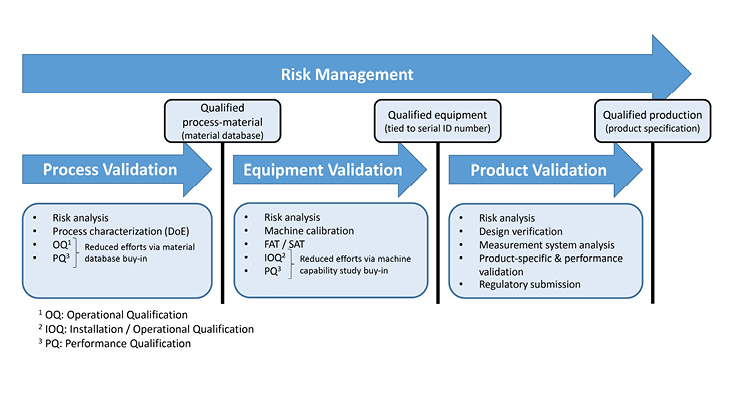
Figure 3: DMP qualification flow chart
Following this approach improves scalability, and enables efficiency improvements as more device types can be produced without repeatedly performing process and equipment validations. We can break down each of the three phases in the flowchart to explore the importance of each.
DMP qualification starts with process validation, in which qualifying the DMP process and the processed material go hand in hand. After process characterization, an operational qualification/process qualification is performed to prove the DMP process’s capability. The process validation sets the material’s acceptance criteria on chemistry, density, mechanical properties, surface roughness, etc.
The second phase of the DMP qualification focuses on validating the equipment—in this case, the DMP machine—and is tied to the printer’s unique serial number. During this phase, a series of test builds are undertaken to assess the robustness and reliability of the printer.
The final phase of DMP qualification focuses on the product-specific qualification. At this phase, the process and equipment have been duly qualified to meet the material and process standards, and the form, function, and performance of the part must be verified or validated. The evaluation of data from the process design stage through commercial production establishes scientific evidence that a process is capable of consistently delivering quality products.2
Integrating AM
Medical device manufacturing is one of the most highly regulated processes, and when choosing to integrate this technology, process characterization and validation are a requirement to ensure success. While materials and hardware were addressed, it’s also important to qualify and validate the supply chain. Securing a logistics ecosystem for reliable sourcing of such new materials at the quality required for healthcare applications can also be a challenging task.
While medical device manufacturers are well-versed in manufacturing, bringing AM into the workflow benefits from the expertise of an experienced partner. Collaborating with an experienced AM solutions company that blends deep expertise with a diverse, broad library of biocompatible materials is invaluable to more easily streamlining these processes.
Whether you choose to take on this activity in-house or with an external partner, having a documented approach and strategy in place alongside a quality management system will ease the path for regulatory submissions, and accelerate time-to-market.
References
Yet with all the amazing innovation, those that are deeply engaged with medical device manufacturing on a daily basis are cognizant of the potential to improve a patient’s life, but also realize we operate in a highly regulated industry. As a result, not only does the device require validation and clearance, the process used to create the device must also be characterized and validated.
Over the past decade, direct metal printing (DMP)—also known as laser powder-bed fusion (LPBF)—has matured significantly and is widely embraced by medical device manufacturers to produce metal components with enhanced functionality and performance to improve clinical outcomes. DMP components for such devices are often manufactured in Ti6Al4V because of the titanium alloy's compelling material properties, such as biocompatibility, high-strength-to-weight ratio, and excellent fatigue properties, along with its extensive current use in the industry. Medical device design and manufacturing is subject to rigid validation/qualification procedures and quality assurance requirements. Hence, stable and capable manufacturing processes are required to drive adoption of the manufacturing technology and the products created from it.
Today, over 100 FDA clearances have been granted for medical devices produced via AM. To help manufacturers navigate the AM process, the FDA released a guidance document in December 2017. This document not only adds credibility to AM as a viable solution for medical device manufacturing, it also offers risk-based, process-driven considerations for the development and manufacture of medical devices using a wide range of AM technologies. It does not, however, act as a stipulative document, thus leaving some flexibility to the process owner for implementation and execution.
Anyone who has integrated AM into a traditional manufacturing workflow can tell you it’s a daunting process. When you integrate AM into a highly regulated medical device workflow, the degree of difficulty skyrockets. Many find the key to an efficient, successful integration of AM is to partner with an experienced organization. There is power in the knowledge and expertise that comes from not only selecting the right solution to address a specific application, but then designing and manufacturing the device, and completing the submission to the FDA.
For example, of the previously mentioned FDA clearances, 3D Systems was involved in a majority of the applications. To date, we’ve produced more than 2 million devices and supported over 100 CE-marked and FDA-cleared devices. A catalyst for this activity is the quality approach, validation strategy, and quality management system we employ, which is aligned to the FDA’s guidance document. The validation strategy can greatly reduce the amount of front-end testing required for a medical device submission, and working with an experienced supplier speeds the time to market and removes risk from the development process. We’ve been able to help manufacturers accelerate their timeline for new market introductions to as little as nine months from concept through FDA clearance.
For those medical device manufacturers looking to integrate DMP into their workflow, or tackle new applications with the technology, it’s important to address several components during process development and validation.
Process Controls
A critical assessment DMP qualification for medical device production relies on a risk-based analysis of the entire DMP process flow—from powder feedstock to printed part. Figure 1 offers a simplified flow chart covering these components.

Figure 1: Simplified DMP process flow including (but not limited to) variables and characteristics of the powder feedstock, process characterization/validation, and the DMP product.
Let’s explore the components of this workflow in more detail.
Powder Feedstock
Quality assurance of the powder feedstock is the first prerequisite for obtaining high-quality and reproducible DMP products. The feedstock quality relies on the powder chemistry and intrinsic powder properties such as particle size distribution (PSD), powder morphology, and the associated flowability. The powder quality is affected by these properties combined with the powder surface chemistry (i.e., moisture and oxygen content). Ultimately, powder quality is a determining factor in the end-use properties of the device and is preeminently important to control adequately.
DMP Process
Exhibiting a stable DMP process is essential to pass the strict validation process, thereby delivering reliable DMP products with excellent reproducible part quality. DMP process stability can be affected by three main process variables: DMP machine condition, DMP parameter set, and/or DMP build file, as illustrated in Figure 1.
Robust DMP Machine Architecture
A stable DMP process with a low build-to-build and machine-to-machine variability starts with a robust machine architecture and appropriate machine calibration procedures. The DMP platform architecture that underlies the DMP Flex 350, DMP Factory 350, and DMP Factory 500 involves a vacuum pre-cycle prior to the printing job that actively removes air and moisture from the build chamber and the powder bed, and fills it afterward with high-purity argon gas. The event of extracting the oxygen and moisture from the powder feed is called the "boiling effect" and is shown in Figure 2.

Figure 2: Boiling effect showing the extraction of oxygen and moisture from the powder feed.1
The efficient/effective vacuum pre-cycle helps achieve extremely low oxygen in the build chamber. Furthermore, the DMP machine’s design ensures no oxygen can leak into the build chamber and, therefore, results in minimal argon consumption during printing. This vacuum chamber concept is key for the desired tight control of the inert atmosphere during printing. Additionally, the vacuum chamber concept helps to eliminate the risks for oxygen pick up by the powder feedstock, resulting in stable powder chemistry and a significant enhancement of the powder reusability of Ti Gr23.1
Poor control of the inert atmosphere (i.e., oxygen and moisture) during the build will lead to an oxygen increase in the printed material. This oxygen enrichment forms a substantial risk for the deterioration of the material’s properties. Therefore, tight control of the inert atmosphere in the build chamber is necessary for extended powder reusability and for obtaining excellent, repeatable mechanical part properties.
DMP Build File
Digital data exchange through software workflows is an essential element of the AM process. Securing digital data exchange and validating software workflows are part of the quality assurance requirements and the DMP qualification process. Developers should seek a software package for AM that enables the user to prepare, optimize, and 3D print high-quality parts—streamlining the workflow from design to post-processing. The software’s job file should contain all parameter data and dimensional data for the part, which can be translated across machines in the same state of maintenance at any location.
In conclusion, quality assurance and monitoring of powder feedstock, combined with a robust DMP process, well-documented machine calibration protocols, and trained machine operators all play a vital role in achieving reliable and repeatable print-to-print quality, yielding fully dense and excellent mechanical properties and surface roughness.
Process Characterization and Qualification
Now that we have all the components defined, we can turn our attention to how the process is characterized and qualified. This begins with the development of a Critical-to-Quality versus Critical Process Parameter (CTQ/CPP) matrix to determine influences and create a pathway to develop studies and establish objective evidence before moving into full process validation. The objective is to ensure all parameters are well-tested and validated while limiting time and incurred cost. The output of the CTQ/CPP matrix is used as input to a process characterization design of experiment and eventually flows into an operational qualification, providing challenge variables and a process window.
To demonstrate this, Figure 3 is the DMP qualification flowchart. The DMP qualification process relies on a risk-based approach and follows a phase-gated flow, passing through process validation, equipment validation, and product validation to achieve qualified product production. The DMP qualification process complies with ASTM F3434.

Figure 3: DMP qualification flow chart
Following this approach improves scalability, and enables efficiency improvements as more device types can be produced without repeatedly performing process and equipment validations. We can break down each of the three phases in the flowchart to explore the importance of each.
DMP qualification starts with process validation, in which qualifying the DMP process and the processed material go hand in hand. After process characterization, an operational qualification/process qualification is performed to prove the DMP process’s capability. The process validation sets the material’s acceptance criteria on chemistry, density, mechanical properties, surface roughness, etc.
The second phase of the DMP qualification focuses on validating the equipment—in this case, the DMP machine—and is tied to the printer’s unique serial number. During this phase, a series of test builds are undertaken to assess the robustness and reliability of the printer.
The final phase of DMP qualification focuses on the product-specific qualification. At this phase, the process and equipment have been duly qualified to meet the material and process standards, and the form, function, and performance of the part must be verified or validated. The evaluation of data from the process design stage through commercial production establishes scientific evidence that a process is capable of consistently delivering quality products.2
Integrating AM
Medical device manufacturing is one of the most highly regulated processes, and when choosing to integrate this technology, process characterization and validation are a requirement to ensure success. While materials and hardware were addressed, it’s also important to qualify and validate the supply chain. Securing a logistics ecosystem for reliable sourcing of such new materials at the quality required for healthcare applications can also be a challenging task.
While medical device manufacturers are well-versed in manufacturing, bringing AM into the workflow benefits from the expertise of an experienced partner. Collaborating with an experienced AM solutions company that blends deep expertise with a diverse, broad library of biocompatible materials is invaluable to more easily streamlining these processes.
Whether you choose to take on this activity in-house or with an external partner, having a documented approach and strategy in place alongside a quality management system will ease the path for regulatory submissions, and accelerate time-to-market.
References
- Beckers, A. et al. On the advances to obtain excellent and repeatable mechanical properties and build quality of LaserForm® Ti gr23 (A) across whole build platform. in 321, 03015 (14th World Conference on Titanium, 2019).
- Westphalen, D., Roth, K. W. & Brodrick, J. Guidance for industry — Process validation: General principles and practices. Food and Drugs Administration (FDA) 45, (2011).