Sam Brusco, Associate Editor03.24.22
Global professional dental solutions maker Dentsply Sirona revealed in late February that it will soon launch Primeprint, an automated, medical-grade 3D printer for dental practices and labs. It’s designed to run the printing process from start to finish—it takes designs based on X-ray images, prints the implants/models, and conducts post-processing tasks like washing, polishing, sanding, and attaching additional components.
“For us dentists, Primeprint turns 3D printing into an efficient application for everyday use, and that’s also great for my patients,” Dr. Mike Skramstad, a Scottsdale, Ariz., dentist, told the press. “The workflow is fully automated, easy to use, as well as end-to-end, so I can both delegate and get the best possible results quickly.”
A recent study¹ of the technology’s ability to make custom occlusal devices using existing maxillary and mandibular intraoral scans showed the Primeprint system’s products only exhibited slight differences from reference designs and accuracy levels similar to that of other 3D printing systems.
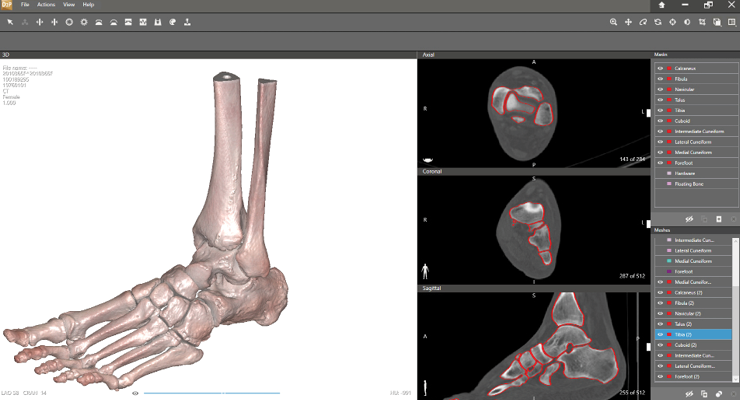
Additive manufacturing (AM), or 3D printing, has been significant for planning and executing orthopedic surgeries as well. 3D printers are smaller, less costly, and more accessible. Open source 3D imaging software to base designs on has facilitated clinical implementation. AM advancements have improved orthopedic surgeons’ approaches to both common and complex cases because advanced imaging models can help build custom surgical guides, instruments, and implants.
“Additive manufacturing is a game-changer when it is beneficial to realize complex shape items,” said Francesco Robotti, technology and business development manager of Lincotek Medical, an Italian design and manufacturing partner for products used in orthopedic, trauma, spinal, and dental applications. “The design-manufacturing freedom enabled by AM cannot be copied with different methods. In this perspective, no matter the quantity needed. The time when additive was intended for prototyping and small series has ended. With decreased part complexity AM may remain the preferred option when a component is requested in thousands of units parcelized across multiple sizes or different geometrical arrangements. A company may save up-front investment in molds and a long time to market to get rid of a portfolio extension. The supply chain flexibility is another key aspect. With increasing demand for customized solutions, AM is a faster responsive method to deliver unique items.”
Some of the world’s largest orthopedic implant manufacturers—including Stryker, DePuy Synthes, Zimmer Biomet, Smith+Nephew, and Medtronic use 3D printing to make implants for knees, hips, spine, ankles, and more.
Spinal implants are among the products most commonly 3D printed. NuVasive, SeaSpine, and Orthofix all rolled out 3D printed porous titanium implants for anterior lumbar interbody fusion (ALIF) last year. Italian firm Tsunami Medical launched nine 3D printed titanium spinal fusion implants since the beginning of last year, and Utah-based Innovasis recently earned U.S. Food and Drug Administration (FDA) clearance for its 3D-printed standalone ALIF system with an HAnano surface modification to provide a rough coating over the 3D-printed scaffold. Lima Corporate also began a partnership with the Hospital for Special Surgery (HSS) to open a 3D Design and Printing Center for Complex Joint Reconstruction Surgery. The FDA-regulated facility is the first of its kind, built to enable faster access to patient-specific implants for highly complex orthopedic ailments.
Outside the clinic walls, 3D printing has also been used as a tool for education, pre-op planning, surgical care, and patient-specific devices and treatments. The hope is one day surgeons can use the tool in a desktop configuration in the clinic or office. But the AM sector has somewhat suffered from overblown expectations. Medical device makers looking to find value using AM need to identify real application opportunities where it can be leveraged.
In December, the FDA published a discussion paper concerning 3D printing medical devices at the point of care (POC), for example, in hospitals and clinics. According to the FDA, the paper isn’t guidance but rather a vehicle to receive public feedback to inform policy development. The paper includes relevant background, terminology, a brief overview of device and 3D printing regulation, and how 3D printing facility capabilities affect device safety and effectiveness. It also points out challenges of POC 3D printed medical devices and offers possible approaches for regulatory oversight under a number of scenarios. It additionally poses questions for public comment.
“The 3D printing of medical devices is at the forefront of innovation and healthcare,” CDRH’s William Maisel, M.D., M.P.H., director of the Office of Product Evaluation and Quality and Ed Margerrison, Ph.D., director of the Office of Science and Engineering Laboratories, commented in an FDA press release. “3D printing at hospitals and other patient-care settings enables health care professionals to quickly create patient-matched devices and anatomical models for surgical planning, as well as many other uses that can help healthcare facilities rapidly respond to patient needs."
Additive Advancements
Orthopedic implants are mostly metal but are sometimes ceramic or made from polyether ether ketone (PEEK) and 3D printed using laser powder bed fusion technology (selective laser melting or electron beam melting) and sometimes made via directed energy deposition. Titanium alloys are the most common material along with cobalt-chrome alloys and stainless steel.
Multi-material 3D printing involves using more than one material in a single print. It’s one of the key capabilities of AM that can utilize its potential beyond that of other manufacturing techniques. It can eliminate the need for assembly, minimize post-processing, and help to efficiently design multi-purpose medical device parts. At present, multi-material printing is only possible using a fused deposition modeling (FDM) printer, where layers of materials are fused together in a pattern to create the product.
Imagine a prosthetic hand—it has to be strong and washable, promoting the use of PETG filament. The fingers also need a good grip, which suggests using TPU. Some of the areas will require very small details, for which PLA might be the best option.
“The advancements in additive manufacturing have allowed companies to use this process for developing complex and precision features utilizing multiple implantable materials, including polymers, a large step forward from past technologies,” said Senja Wahlman, Sales Account Manager at Able Medical Devices, a Gwinn, Mich.-based developer of precision-machined surgical implants and instruments. “These advancements allow for more precise geometries and cut down on lead times.”
The process can be challenging, however. A complex design might need material changes—hundreds or even thousands of them—within a single print, making the process dramatically slower. Switching materials isn’t simply swapping the spool, either. PLA and TPU need very different slicer settings to print well, requiring changing the nozzle and bed temperatures, print speed, retraction settings, etc. All of one material also needs to be purged before printing in another.
Binder jetting is an additive manufacturing process where the printhead deposits a liquid binding agent on a thin layer of powder particles. The binder acts like an ink as it moves across the layers of powder, forming the final product. The particles can be metal, sand, ceramics, or composites to build parts and tooling. The process was first developed at the Massachussetts Institute of Technology in the early 1990s. Two years later, the first commercial binder jet 3D printer for metals hit the market.
Depending on its application, some materials require minimal or no post-processing. Other materials are cured and sintered after printing for densities over 97 percent. Parts are sometimes infiltrated with other material for the desired matrix or composite material.
“We focus on binder-jet technology, which lets us leverage our expertise in sintering, gained from over 50 years of innovation in metal injection molding,” said David Hotter, senior director of business development at ATW Company’s Parmatech, a supplier of custom manufactured metal injection molded (MIM) components. “We have been refining the process for over three years and have been encouraged at the productivity increases realized with our equipment. Initially, it was thought binder-jetting would not compete at higher volumes, particularly for smaller parts that could also be produced with metal injection molding. The thought was we could develop parts using additive manufacturing but as production volumes increased, we would need to transition to metal injection molding to remain cost competitive. We have since been able to refine the process and prove that binder-jetting is a viable high-volume production process that can remain cost competitive with volumes approaching 100,000 parts a year.”
Selective laser sintering (SLS) was one of the first additive manufacturing techniques, first developed in the mid-1980s by University of Texas at Austin’s Dr. Carl Deckard and Dr. Joe Beaman. SLS uses a high-power laser to sinter small particles of polymer powder into a solid structure using a 3D model. The method has been adapted for a range of materials—plastics, metals, glass, ceramics, and various composites. Today, the technologies are collectively named powder bed fusion.
SLS 3D printing has been popular for engineers and manufacturers for decades because of its low cost per part, high productivity, and use of established materials. It’s been used for rapid prototyping as well as small-batch, bridge, or custom manufacturing. Recent machinery, materials, and software innovations have made SLS printing more available to a wider range of businesses.
“Our free-flowing polycaprolactone powder, RESOMER PrintPowder C 212 F, was launched in 2020 and was the world’s first bioresorbable powder suitable for medical implants,” said Dr. Thiago Borges, manager for customer projects at Evonik Health Care, a partner with pharmaceutical, medical device and nutraceutical companies for specialty chemical solutions. “Since then, new materials have been added to the portfolio, including new polylactide and polydioxanone-based powders, which are available for sampling and customer evaluation. Another important addition to our portfolio was RESOMER Filament in 2020. This is available in the four standard grades poly(L-lactide), poly (L-lactide-co-glycolide), poly(caprolactone), and polydioxanone. Customized materials allowing tailored mechanical properties and degradation time for orthopedic, dental, or soft tissue applications are also available on request.”
RESOMER PrintPowder C 212 F is also used in fused filament fabrication (FFF). This process feeds a continuous filament composed of thermoplastic material through a heated printer extruder head and deposited to form layers. The extruder head usually moves in two directions to create one layer at a time before adjusting vertically to start a new layer. A large variety of materials can be used in FFF and it also allows fast printing time and has multiple printer manufacturers. Printing using FFF is flexible and allows small overhangs using support from the bottom layers as well as more detailed parts and complicated features die to smaller extruder nozzles and bead width.
Nickel titanium (Nitinol) is a metal alloy with the unique ability to change shape up to 8 percent strain and still recover back to its original shape (shape memory). The mechanically active alloy received its name in 1959 at the Naval Ordinal Laboratory, its original place of discovery.
Depending on its composition and temperature, Nitinol either exhibits shape memory or “superelastic” properties. When heated to body temperature for biomedical applications, shape memory Nitinol will revert back to its original geometry. When activation is tailored to body temperature, shape change can occur once implanted in the body. Nitinol has a long track record of use in medtech for self-expanding stents, guide wires, orthodontic wires, and orthopedic staples.
However, it can be a challenge to use it in additive manufacturing processes.
“Nitinol exhibits work hardening in conventional manufacturing processes and is extremely difficult to 3D print due to changes in chemistry during the AM part lifecycle,” said Gaurav Lalwani, Global Medical Applications Engineering Lead at Carpenter Additive, a Philadelphia, Pa.-based fully integrated metal additive AM partner. “Effective translation of shape memory and superelastic properties in a 3D-printed Nitinol component is a challenge. Carpenter Additive offers EIGA (electrode induction gas atomization) Nitinol powder production that maintains high nickel levels during atomization. This powder, used in-house by our AM engineers and coupled with custom processes, exhibited up to 6 percent of shape memory strain recovery in 3D-printed bone staples.”
Noteworthy Projects
Some orthopedic implants require good strength-to-weight ratios, desirable shock/sound absorption, and larger surface areas. In this case, the design may call for a lattice structure, which is comprised of micro-architectures with a network of nodes and beams (or struts). A lattice geometry significantly reduces weight while retaining integrity and allowing a larger degree of control over certain aspects. The interlinking portions can boost various performance areas and use less material without weakening the implant or compromising integrity.
Lattices are common in 3D-printed spine implants because they encourage better bone ingrowth and faster healing without stress shielding. The porous grid architecture can be changed according to individual implant needs. The design also helps reduce subsidence, pseudoarthrosis, and implant failure. The applications of lattice and porous structures are becoming more common for weight-bearing implants in multiple areas of the body.
“We worked on a shoulder project that leveraged two different lattice structures we helped design,” said Brian McLaughlin, founder and CEO of Amplify Additive, a Scarborough, Maine-based company focused on metal additive manufacturing using GE Additive’s Arcam Q10plus EBM technology. “One lattice was a trabecular with a roughness factor that made the implant stickier for initial fixation and the other lattice design was more open to mimic more cancellous bone. The uniqueness of this device is there is really no other way to manufacture this type of design.”
In November 2020, the FDA granted 510(k) clearance to the Vantage Ankle PSI patient-specific total ankle surgical planning and 3D printed instruments. The tool includes pre-surgical planning and a patient-specific 3D printed instrument set to guide resections in the tibia and talus for total ankle replacement surgery using orthopedic device maker Exactech’s Vantage total ankle system. Exactech collaborated with additive manufacturing solutions provider 3D Systems to develop the product.
According to 3D Systems, Vantage Ankle PSI is the only solution to facilitate direct patient-specific osteotomies in the ankle. It features a large footprint to reliably seat the guide on the bone, improves visibility for alignment, and has a corrugated design on cutting slots to aid surgical irrigation. Soft tissue offsets preserve the periosteum, the outer fibrous bone layer that aids in healing and recovery.
“3D Systems’ Vantage Ankle PSI is noteworthy as it demonstrates the value of additive manufacturing in patient-specific applications, 3D Systems’ vices president and general manager of medical devices Gautam Gupta told ODT. “The Vantage Ankle PSI consists of pre-operative planning, surgical guides, and an anatomic model for total ankle arthroplasty (TAA) procedures. This project stands out, as it provides an innovative product to surgeons for a complex orthopedic surgery. Vantage Ankle PSI surgical guides differ from other products on the market by having cut-through slots and other features aimed at reducing surgery complexity and operation times.”
When to 3D Print?
Though additive manufacturing technology has found use in orthopedic device manufacturing, it’s important to know its most appropriate use cases—otherwise production may be slowed or become too costly. The technology is still best suited for prototyping and low-volume manufacturing. It’s also effective for making parts that have intricate details, creating unique and complex components that are difficult to fabricate using conventional processes like CNC machining.
For a long time, companies have viewed AM as a “cure-all” technology rather than another tool in their toolbox. They may not be maximizing its potential and underusing it in the wrong places. For example, post-processing is still a necessary step for many orthopedic devices when tolerances need to be tight or surfaces still require polishing and finishing. Accuracy and polishing may bring about cost issues and drive AM from many applications where it might be otherwise preferred.
“Additive manufacturing with metals is preferable to other methods in several situations: for rapid prototyping; when components are relatively small and of complex geometry; and for custom implants used in complex reconstructions,” said David Anderson, marketing and business development leader for SITES Medical, a Columbia City, Ind.-based orthopedic technology development company. “While we believe there are good applications of additive manufacturing, they are limited. For example, for many applications where components are relatively large and markets are price sensitive, such as hips or knees, implants made with AM cannot be made at an appropriate cost point. There are also some design limitations to AM. AM doesn’t permit the build of a knee femoral component with a CoCr articulating surface and a Ti ingrowth material. AM also produces implants with the strength of cast components, which sometimes isn’t sufficient for the application (e.g., hip stems) or requires an otherwise unnecessary compromise (e.g., thicker tibial plates require sacrifices in bone resection level or poly thickness).”
Reference
“For us dentists, Primeprint turns 3D printing into an efficient application for everyday use, and that’s also great for my patients,” Dr. Mike Skramstad, a Scottsdale, Ariz., dentist, told the press. “The workflow is fully automated, easy to use, as well as end-to-end, so I can both delegate and get the best possible results quickly.”
A recent study¹ of the technology’s ability to make custom occlusal devices using existing maxillary and mandibular intraoral scans showed the Primeprint system’s products only exhibited slight differences from reference designs and accuracy levels similar to that of other 3D printing systems.
Additive manufacturing (AM), or 3D printing, has been significant for planning and executing orthopedic surgeries as well. 3D printers are smaller, less costly, and more accessible. Open source 3D imaging software to base designs on has facilitated clinical implementation. AM advancements have improved orthopedic surgeons’ approaches to both common and complex cases because advanced imaging models can help build custom surgical guides, instruments, and implants.
“Additive manufacturing is a game-changer when it is beneficial to realize complex shape items,” said Francesco Robotti, technology and business development manager of Lincotek Medical, an Italian design and manufacturing partner for products used in orthopedic, trauma, spinal, and dental applications. “The design-manufacturing freedom enabled by AM cannot be copied with different methods. In this perspective, no matter the quantity needed. The time when additive was intended for prototyping and small series has ended. With decreased part complexity AM may remain the preferred option when a component is requested in thousands of units parcelized across multiple sizes or different geometrical arrangements. A company may save up-front investment in molds and a long time to market to get rid of a portfolio extension. The supply chain flexibility is another key aspect. With increasing demand for customized solutions, AM is a faster responsive method to deliver unique items.”
Some of the world’s largest orthopedic implant manufacturers—including Stryker, DePuy Synthes, Zimmer Biomet, Smith+Nephew, and Medtronic use 3D printing to make implants for knees, hips, spine, ankles, and more.
Spinal implants are among the products most commonly 3D printed. NuVasive, SeaSpine, and Orthofix all rolled out 3D printed porous titanium implants for anterior lumbar interbody fusion (ALIF) last year. Italian firm Tsunami Medical launched nine 3D printed titanium spinal fusion implants since the beginning of last year, and Utah-based Innovasis recently earned U.S. Food and Drug Administration (FDA) clearance for its 3D-printed standalone ALIF system with an HAnano surface modification to provide a rough coating over the 3D-printed scaffold. Lima Corporate also began a partnership with the Hospital for Special Surgery (HSS) to open a 3D Design and Printing Center for Complex Joint Reconstruction Surgery. The FDA-regulated facility is the first of its kind, built to enable faster access to patient-specific implants for highly complex orthopedic ailments.
Outside the clinic walls, 3D printing has also been used as a tool for education, pre-op planning, surgical care, and patient-specific devices and treatments. The hope is one day surgeons can use the tool in a desktop configuration in the clinic or office. But the AM sector has somewhat suffered from overblown expectations. Medical device makers looking to find value using AM need to identify real application opportunities where it can be leveraged.
In December, the FDA published a discussion paper concerning 3D printing medical devices at the point of care (POC), for example, in hospitals and clinics. According to the FDA, the paper isn’t guidance but rather a vehicle to receive public feedback to inform policy development. The paper includes relevant background, terminology, a brief overview of device and 3D printing regulation, and how 3D printing facility capabilities affect device safety and effectiveness. It also points out challenges of POC 3D printed medical devices and offers possible approaches for regulatory oversight under a number of scenarios. It additionally poses questions for public comment.
“The 3D printing of medical devices is at the forefront of innovation and healthcare,” CDRH’s William Maisel, M.D., M.P.H., director of the Office of Product Evaluation and Quality and Ed Margerrison, Ph.D., director of the Office of Science and Engineering Laboratories, commented in an FDA press release. “3D printing at hospitals and other patient-care settings enables health care professionals to quickly create patient-matched devices and anatomical models for surgical planning, as well as many other uses that can help healthcare facilities rapidly respond to patient needs."
Additive Advancements
Orthopedic implants are mostly metal but are sometimes ceramic or made from polyether ether ketone (PEEK) and 3D printed using laser powder bed fusion technology (selective laser melting or electron beam melting) and sometimes made via directed energy deposition. Titanium alloys are the most common material along with cobalt-chrome alloys and stainless steel.
Multi-material 3D printing involves using more than one material in a single print. It’s one of the key capabilities of AM that can utilize its potential beyond that of other manufacturing techniques. It can eliminate the need for assembly, minimize post-processing, and help to efficiently design multi-purpose medical device parts. At present, multi-material printing is only possible using a fused deposition modeling (FDM) printer, where layers of materials are fused together in a pattern to create the product.
Imagine a prosthetic hand—it has to be strong and washable, promoting the use of PETG filament. The fingers also need a good grip, which suggests using TPU. Some of the areas will require very small details, for which PLA might be the best option.
“The advancements in additive manufacturing have allowed companies to use this process for developing complex and precision features utilizing multiple implantable materials, including polymers, a large step forward from past technologies,” said Senja Wahlman, Sales Account Manager at Able Medical Devices, a Gwinn, Mich.-based developer of precision-machined surgical implants and instruments. “These advancements allow for more precise geometries and cut down on lead times.”
The process can be challenging, however. A complex design might need material changes—hundreds or even thousands of them—within a single print, making the process dramatically slower. Switching materials isn’t simply swapping the spool, either. PLA and TPU need very different slicer settings to print well, requiring changing the nozzle and bed temperatures, print speed, retraction settings, etc. All of one material also needs to be purged before printing in another.
Binder jetting is an additive manufacturing process where the printhead deposits a liquid binding agent on a thin layer of powder particles. The binder acts like an ink as it moves across the layers of powder, forming the final product. The particles can be metal, sand, ceramics, or composites to build parts and tooling. The process was first developed at the Massachussetts Institute of Technology in the early 1990s. Two years later, the first commercial binder jet 3D printer for metals hit the market.
Depending on its application, some materials require minimal or no post-processing. Other materials are cured and sintered after printing for densities over 97 percent. Parts are sometimes infiltrated with other material for the desired matrix or composite material.
“We focus on binder-jet technology, which lets us leverage our expertise in sintering, gained from over 50 years of innovation in metal injection molding,” said David Hotter, senior director of business development at ATW Company’s Parmatech, a supplier of custom manufactured metal injection molded (MIM) components. “We have been refining the process for over three years and have been encouraged at the productivity increases realized with our equipment. Initially, it was thought binder-jetting would not compete at higher volumes, particularly for smaller parts that could also be produced with metal injection molding. The thought was we could develop parts using additive manufacturing but as production volumes increased, we would need to transition to metal injection molding to remain cost competitive. We have since been able to refine the process and prove that binder-jetting is a viable high-volume production process that can remain cost competitive with volumes approaching 100,000 parts a year.”
Selective laser sintering (SLS) was one of the first additive manufacturing techniques, first developed in the mid-1980s by University of Texas at Austin’s Dr. Carl Deckard and Dr. Joe Beaman. SLS uses a high-power laser to sinter small particles of polymer powder into a solid structure using a 3D model. The method has been adapted for a range of materials—plastics, metals, glass, ceramics, and various composites. Today, the technologies are collectively named powder bed fusion.
SLS 3D printing has been popular for engineers and manufacturers for decades because of its low cost per part, high productivity, and use of established materials. It’s been used for rapid prototyping as well as small-batch, bridge, or custom manufacturing. Recent machinery, materials, and software innovations have made SLS printing more available to a wider range of businesses.
“Our free-flowing polycaprolactone powder, RESOMER PrintPowder C 212 F, was launched in 2020 and was the world’s first bioresorbable powder suitable for medical implants,” said Dr. Thiago Borges, manager for customer projects at Evonik Health Care, a partner with pharmaceutical, medical device and nutraceutical companies for specialty chemical solutions. “Since then, new materials have been added to the portfolio, including new polylactide and polydioxanone-based powders, which are available for sampling and customer evaluation. Another important addition to our portfolio was RESOMER Filament in 2020. This is available in the four standard grades poly(L-lactide), poly (L-lactide-co-glycolide), poly(caprolactone), and polydioxanone. Customized materials allowing tailored mechanical properties and degradation time for orthopedic, dental, or soft tissue applications are also available on request.”
RESOMER PrintPowder C 212 F is also used in fused filament fabrication (FFF). This process feeds a continuous filament composed of thermoplastic material through a heated printer extruder head and deposited to form layers. The extruder head usually moves in two directions to create one layer at a time before adjusting vertically to start a new layer. A large variety of materials can be used in FFF and it also allows fast printing time and has multiple printer manufacturers. Printing using FFF is flexible and allows small overhangs using support from the bottom layers as well as more detailed parts and complicated features die to smaller extruder nozzles and bead width.
Nickel titanium (Nitinol) is a metal alloy with the unique ability to change shape up to 8 percent strain and still recover back to its original shape (shape memory). The mechanically active alloy received its name in 1959 at the Naval Ordinal Laboratory, its original place of discovery.
Depending on its composition and temperature, Nitinol either exhibits shape memory or “superelastic” properties. When heated to body temperature for biomedical applications, shape memory Nitinol will revert back to its original geometry. When activation is tailored to body temperature, shape change can occur once implanted in the body. Nitinol has a long track record of use in medtech for self-expanding stents, guide wires, orthodontic wires, and orthopedic staples.
However, it can be a challenge to use it in additive manufacturing processes.
“Nitinol exhibits work hardening in conventional manufacturing processes and is extremely difficult to 3D print due to changes in chemistry during the AM part lifecycle,” said Gaurav Lalwani, Global Medical Applications Engineering Lead at Carpenter Additive, a Philadelphia, Pa.-based fully integrated metal additive AM partner. “Effective translation of shape memory and superelastic properties in a 3D-printed Nitinol component is a challenge. Carpenter Additive offers EIGA (electrode induction gas atomization) Nitinol powder production that maintains high nickel levels during atomization. This powder, used in-house by our AM engineers and coupled with custom processes, exhibited up to 6 percent of shape memory strain recovery in 3D-printed bone staples.”
Noteworthy Projects
Some orthopedic implants require good strength-to-weight ratios, desirable shock/sound absorption, and larger surface areas. In this case, the design may call for a lattice structure, which is comprised of micro-architectures with a network of nodes and beams (or struts). A lattice geometry significantly reduces weight while retaining integrity and allowing a larger degree of control over certain aspects. The interlinking portions can boost various performance areas and use less material without weakening the implant or compromising integrity.
Lattices are common in 3D-printed spine implants because they encourage better bone ingrowth and faster healing without stress shielding. The porous grid architecture can be changed according to individual implant needs. The design also helps reduce subsidence, pseudoarthrosis, and implant failure. The applications of lattice and porous structures are becoming more common for weight-bearing implants in multiple areas of the body.
“We worked on a shoulder project that leveraged two different lattice structures we helped design,” said Brian McLaughlin, founder and CEO of Amplify Additive, a Scarborough, Maine-based company focused on metal additive manufacturing using GE Additive’s Arcam Q10plus EBM technology. “One lattice was a trabecular with a roughness factor that made the implant stickier for initial fixation and the other lattice design was more open to mimic more cancellous bone. The uniqueness of this device is there is really no other way to manufacture this type of design.”
In November 2020, the FDA granted 510(k) clearance to the Vantage Ankle PSI patient-specific total ankle surgical planning and 3D printed instruments. The tool includes pre-surgical planning and a patient-specific 3D printed instrument set to guide resections in the tibia and talus for total ankle replacement surgery using orthopedic device maker Exactech’s Vantage total ankle system. Exactech collaborated with additive manufacturing solutions provider 3D Systems to develop the product.
According to 3D Systems, Vantage Ankle PSI is the only solution to facilitate direct patient-specific osteotomies in the ankle. It features a large footprint to reliably seat the guide on the bone, improves visibility for alignment, and has a corrugated design on cutting slots to aid surgical irrigation. Soft tissue offsets preserve the periosteum, the outer fibrous bone layer that aids in healing and recovery.
“3D Systems’ Vantage Ankle PSI is noteworthy as it demonstrates the value of additive manufacturing in patient-specific applications, 3D Systems’ vices president and general manager of medical devices Gautam Gupta told ODT. “The Vantage Ankle PSI consists of pre-operative planning, surgical guides, and an anatomic model for total ankle arthroplasty (TAA) procedures. This project stands out, as it provides an innovative product to surgeons for a complex orthopedic surgery. Vantage Ankle PSI surgical guides differ from other products on the market by having cut-through slots and other features aimed at reducing surgery complexity and operation times.”
When to 3D Print?
Though additive manufacturing technology has found use in orthopedic device manufacturing, it’s important to know its most appropriate use cases—otherwise production may be slowed or become too costly. The technology is still best suited for prototyping and low-volume manufacturing. It’s also effective for making parts that have intricate details, creating unique and complex components that are difficult to fabricate using conventional processes like CNC machining.
For a long time, companies have viewed AM as a “cure-all” technology rather than another tool in their toolbox. They may not be maximizing its potential and underusing it in the wrong places. For example, post-processing is still a necessary step for many orthopedic devices when tolerances need to be tight or surfaces still require polishing and finishing. Accuracy and polishing may bring about cost issues and drive AM from many applications where it might be otherwise preferred.
“Additive manufacturing with metals is preferable to other methods in several situations: for rapid prototyping; when components are relatively small and of complex geometry; and for custom implants used in complex reconstructions,” said David Anderson, marketing and business development leader for SITES Medical, a Columbia City, Ind.-based orthopedic technology development company. “While we believe there are good applications of additive manufacturing, they are limited. For example, for many applications where components are relatively large and markets are price sensitive, such as hips or knees, implants made with AM cannot be made at an appropriate cost point. There are also some design limitations to AM. AM doesn’t permit the build of a knee femoral component with a CoCr articulating surface and a Ti ingrowth material. AM also produces implants with the strength of cast components, which sometimes isn’t sufficient for the application (e.g., hip stems) or requires an otherwise unnecessary compromise (e.g., thicker tibial plates require sacrifices in bone resection level or poly thickness).”
Reference