Maria Shepherd, President and Founder, Medi-Vantage02.17.23
In its first in-person, face-to-face meeting since 2019, AdvaMed brought top medtech executives and innovators to Boston last October to share strategies for the future and discuss critical topics. The MedTech Conference is similar to a family reunion for the medtech industry, with many not seen for years who want to share their “stories” for medical and orthopedic device development, manufacture, and distribution best practices. The global community can meet and greet new investors and partners, while hearing of new ideas and innovations. In 2023, the conference is scheduled to take place in Anaheim, Calif.
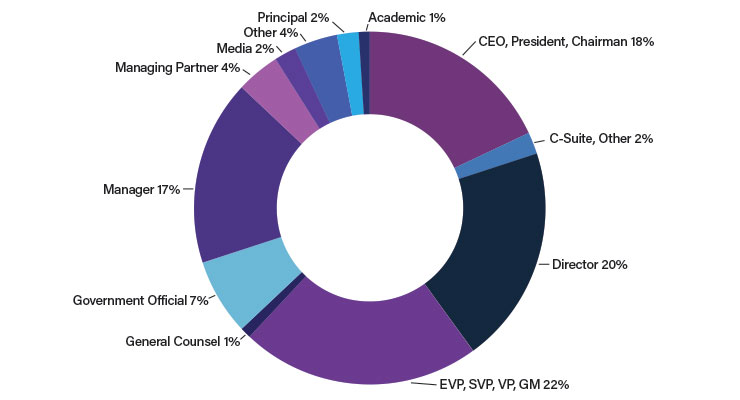
CEOs are one of the big draws for me at The Medtech Conference. AdvaMed stated nearly 50% of the attendees at this conference were executive leaders from 45 different countries (Tables 1&2).2,3 The CEO Unplugged panels were of special interest as these panels featured discussions about topics such as addressing the challenges of the pandemic to ensure medical device firms were able to keep their supply chains resilient and their sales forces relevant and in hospitals, while also continuing to work with physicians. In addition, they spoke about utilizing new sources of data to form strategies to reach their goals, trends in mergers and acquisitions, successes and challenges in the ongoing quest for fundraising, top changes shaping our industry, leading during times of change and difficulty, the challenges of true innovation in the medical device space, and the quest for authentic digital innovation.
We discussed AI and how to apply it to the next big expansion of EU medtech. The delegation told me about the Advanced Manufacturing Centre—a strategic national initiative supported by the government of Ireland through IDA Ireland, dedicated to creating an industry-led national center that empowers manufacturers based in Ireland to “access, adopt and accelerate” innovative digital technologies, which explain real world challenges and create future competitive advantages.6
IDA Ireland’s vision is to position the Irish manufacturing base at the front of digital transformation to ensure Ireland is recognized globally as having a vital, cooperative, competitive, and digitally empowered business center, ideally capable of producing the next generation of industry.
ResMed CEO Mick Farrell introduced his company to attendees; it manufactures medical capital equipment linked to the cloud to treat sleep apnea and COPD. The technology is called AirSense and AirCurve, and one of its most vital sales tools is the myAir app.7
CPAP is a big market (Table 3). Approximately 26% of adults aged 30 to 70 years old have obstructive sleep apnea because as we grow older, the percentage of people with sleep apnea increases significantly.8 If ever there was a space that needed disrupting, it's the CPAP space.
Mick told attendees his team shifted from examining patient data with the decades old “How do we monetize patient data?” lens to one that returns important data to patients. Now ResMed patients are excited to use their CPAP and see what their sleep scores are using the myAir app. Through this process of gamifying patient sleep scores, ResMed retains patient data and leverages it for analytical purposes that could yield important patient outcomes and reduce costs in the future, while also continuing to improve ResMed’s competitive advantage in the fiercely aggressive CPAP sales space.
Rachel cited the ability of the mymobility app to track data after a knee procedure to improve outcomes and reduce costs. mymobility represents a Zimmer Biomet partnership with Apple Watch to provide a digital care management platform that uses an iPhone to deliver support and guidance to patients through a connected experience. Similar to the ResMed example, the knee implant market is a very competitive space and the mymobility app is one that has the potential to protect (or grow) Zimmer Biomet market share.
In November 2022, Zimmer Biomet announced one-year data from the mymobility clinical study at 2022 AAHKS Annual Meeting.9 The study (n=401) showed similar outcomes as traditional surgery and resulted in reduced outpatient physical therapy and surgery-related emergency department visits after knee replacement. Both could lead to lower costs of care, although a comparative analysis of costs was not part of the study design.
Don't let conventional wisdom define your strategies. Sometimes, it's hard to look outside the lens of your internal landscapes, but that's exactly what the smart strategic thinkers at ResMed did and what you too can do.
References
Maria Shepherd has more than 20 years of leadership experience in marketing in small startups and top-tier companies. After her industry career, she founded Medi-Vantage, which provides marketing and business strategy and innovation research for the medical device industry. Shepherd can be reached at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.
Why This Is Important
The Advanced Medical Technology Association (AdvaMed) is a global trade association of medical device companies that develop, create, and sell high-quality, innovative medical technology; these companies are committed to improving the lives of patients around the world.1 More than 100 sessions brought new perspectives to a very diverse audience about investing, hospital trends, new marketing trends, and the advances being made in AI, cybersecurity, and global trade.CEOs are one of the big draws for me at The Medtech Conference. AdvaMed stated nearly 50% of the attendees at this conference were executive leaders from 45 different countries (Tables 1&2).2,3 The CEO Unplugged panels were of special interest as these panels featured discussions about topics such as addressing the challenges of the pandemic to ensure medical device firms were able to keep their supply chains resilient and their sales forces relevant and in hospitals, while also continuing to work with physicians. In addition, they spoke about utilizing new sources of data to form strategies to reach their goals, trends in mergers and acquisitions, successes and challenges in the ongoing quest for fundraising, top changes shaping our industry, leading during times of change and difficulty, the challenges of true innovation in the medical device space, and the quest for authentic digital innovation.
The Emerald Isle
I had a chance to speak to representatives from IDA Ireland; the region bills itself as Europe’s premier location for medtech innovation (for good reason). During the discussion, I learned there are more than 300 medical technology companies in Ireland and they have a 70-plus year record of working with medtech. IDA Ireland helps companies transition to industry 4.0 and to digitization.We discussed AI and how to apply it to the next big expansion of EU medtech. The delegation told me about the Advanced Manufacturing Centre—a strategic national initiative supported by the government of Ireland through IDA Ireland, dedicated to creating an industry-led national center that empowers manufacturers based in Ireland to “access, adopt and accelerate” innovative digital technologies, which explain real world challenges and create future competitive advantages.6
IDA Ireland’s vision is to position the Irish manufacturing base at the front of digital transformation to ensure Ireland is recognized globally as having a vital, cooperative, competitive, and digitally empowered business center, ideally capable of producing the next generation of industry.
Capital Equipment Rebound?
At the conference within the CEOs Unplugged panels, there were glimmers of hope that first-class, state-of-the-art strategizing could reverse capital equipment sales trends, which have been decimated since the beginning of the pandemic.ResMed CEO Mick Farrell introduced his company to attendees; it manufactures medical capital equipment linked to the cloud to treat sleep apnea and COPD. The technology is called AirSense and AirCurve, and one of its most vital sales tools is the myAir app.7
CPAP is a big market (Table 3). Approximately 26% of adults aged 30 to 70 years old have obstructive sleep apnea because as we grow older, the percentage of people with sleep apnea increases significantly.8 If ever there was a space that needed disrupting, it's the CPAP space.
Mick told attendees his team shifted from examining patient data with the decades old “How do we monetize patient data?” lens to one that returns important data to patients. Now ResMed patients are excited to use their CPAP and see what their sleep scores are using the myAir app. Through this process of gamifying patient sleep scores, ResMed retains patient data and leverages it for analytical purposes that could yield important patient outcomes and reduce costs in the future, while also continuing to improve ResMed’s competitive advantage in the fiercely aggressive CPAP sales space.
What About the Orthopedic Market?
Rachel Ellingson, Zimmer Biomet SVP of corporate strategy, was superb on her CEO panel talking about what Zimmer Biomet did before and during COVID to be able to create a center of technology excellence. The Zimmer Biomet shift to an R&D center of technology excellence was a fortuitous decision made just in time before COVID without any advanced knowledge of the pandemic. It was a huge opportunity for Zimmer Biomet, but also a big challenge, explained Rachel. It created a centralized system where, for example, new technology like AI in devices can inform better care for healthcare providers and patients.Rachel cited the ability of the mymobility app to track data after a knee procedure to improve outcomes and reduce costs. mymobility represents a Zimmer Biomet partnership with Apple Watch to provide a digital care management platform that uses an iPhone to deliver support and guidance to patients through a connected experience. Similar to the ResMed example, the knee implant market is a very competitive space and the mymobility app is one that has the potential to protect (or grow) Zimmer Biomet market share.
In November 2022, Zimmer Biomet announced one-year data from the mymobility clinical study at 2022 AAHKS Annual Meeting.9 The study (n=401) showed similar outcomes as traditional surgery and resulted in reduced outpatient physical therapy and surgery-related emergency department visits after knee replacement. Both could lead to lower costs of care, although a comparative analysis of costs was not part of the study design.
The Medi-Vantage Perspective
Even through the pandemic, good strategies were developed by smart people in the medtech space. All it required was finding a different perspective, or examining the problem differently than the status quo.Don't let conventional wisdom define your strategies. Sometimes, it's hard to look outside the lens of your internal landscapes, but that's exactly what the smart strategic thinkers at ResMed did and what you too can do.
References
- bit.ly/mpo230101
- bit.ly/mpo230102
- bit.ly/mpo230103
- bit.ly/mpo230104
- bit.ly/mpo230105
- bit.ly/mpo230106
- apple.co/3XxDAHr
- bit.ly/mpo230109
- bit.ly/odt230109
Maria Shepherd has more than 20 years of leadership experience in marketing in small startups and top-tier companies. After her industry career, she founded Medi-Vantage, which provides marketing and business strategy and innovation research for the medical device industry. Shepherd can be reached at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.