Medacta09.16.20
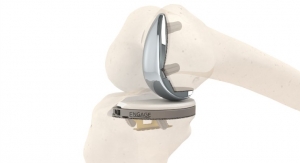
Medacta announced the first surgery utilizing its MOTO Lateral Partial Knee System, which has recently received CE marking and FDA clearance. The surgery was performed by J. Mandume Kerina, M.D., from Lady Lake, FL (USA) in early August.
MOTO Lateral is a compartment-specific, fixed-bearing implant that allows for the treatment of osteoarthritis localized on the lateral femoral and tibial condyles. This device is part of the MOTO Partial Knee System, which also includes MOTO Medial (launched in 2017), and MOTO PFJ, a new Patello-Femoral Joint implant that will be available at the beginning of 2021.
The MOTO System provides surgeons with a comprehensive range of options to resurface only the affected portion of the patient's knee. Potential benefits of this minimally invasive procedure include a shorter hospital stay, faster recovery and better knee function compared to a total knee arthroplasty. All MOTO System components feature an anatomic and compartment-specific design, based on Medacta's MyBody anthropometric database, which consists of more than 45,000 CT and MRI knee scans. This optimizes anatomic coverage and size range for the broadest range of patient populations.
The MOTO instrument platform allows for precise bone resections and the control of flexion-extension gap balancing at each step of the surgery in order to precisely resurface the affected medial or lateral compartment of the patient's knee. In keeping with its commitment to surgeon education, Medacta's M.O.R.E. Institute has also created a comprehensive Education Program dedicated to the MOTO Platform, with the goal of teaching surgeons the principles of the innovative surgical procedure using this system.
"I have been using the MOTO Medial UKA system since its release, with excellent results," said Dr. Kerina. "Now, with the addition of the MOTO Lateral to the system, I can cover almost all my tibiofemoral unicompartmental knee arthroplasties. Being able to provide my patients with a wider range of arthroplasty options to treat their specific pathology has allowed me to greatly expand my practice. I am very excited about what the MOTO system will become when the last puzzle piece, the MOTO PFJ, will be released early next year, allowing me to treat also the patellofemoral osteoarthritis. The system offers a comprehensive range of options aiming to intra-op flexibility and patients' well-being."
MOTO PFJ (Patello-Femoral Joint), with its anticipated availability in 2021, will allow for the treatment of patellofemoral osteoarthritis without compromising the non-affected tibiofemoral knee compartments. This implant features an onlay-anterior and inlay-distal design with a broad-anterior and narrow-distal shape, conceived to restore a painless patellar movement throughout the whole articular range of motion. The dedicated surgical technique and instrumentation guide every step of the procedure, utilizing the anterior cortex as reference and completely avoiding intramedullary canal violation or any free-hand preparation.
The introduction of these innovative implants and their cutting-edge concepts is possible through the M.O.R.E. Excellence Clinical Program, which defines the steps and milestones applicable to all Medacta products ahead of their full release. Under this program, Medacta releases new products on a restricted basis to conduct voluntary clinical programs in order to further document their safety and efficacy.
Discover more about Medacta's knee portfolio at knee.medacta.com.
MOTO Lateral is a compartment-specific, fixed-bearing implant that allows for the treatment of osteoarthritis localized on the lateral femoral and tibial condyles. This device is part of the MOTO Partial Knee System, which also includes MOTO Medial (launched in 2017), and MOTO PFJ, a new Patello-Femoral Joint implant that will be available at the beginning of 2021.
The MOTO System provides surgeons with a comprehensive range of options to resurface only the affected portion of the patient's knee. Potential benefits of this minimally invasive procedure include a shorter hospital stay, faster recovery and better knee function compared to a total knee arthroplasty. All MOTO System components feature an anatomic and compartment-specific design, based on Medacta's MyBody anthropometric database, which consists of more than 45,000 CT and MRI knee scans. This optimizes anatomic coverage and size range for the broadest range of patient populations.
The MOTO instrument platform allows for precise bone resections and the control of flexion-extension gap balancing at each step of the surgery in order to precisely resurface the affected medial or lateral compartment of the patient's knee. In keeping with its commitment to surgeon education, Medacta's M.O.R.E. Institute has also created a comprehensive Education Program dedicated to the MOTO Platform, with the goal of teaching surgeons the principles of the innovative surgical procedure using this system.
"I have been using the MOTO Medial UKA system since its release, with excellent results," said Dr. Kerina. "Now, with the addition of the MOTO Lateral to the system, I can cover almost all my tibiofemoral unicompartmental knee arthroplasties. Being able to provide my patients with a wider range of arthroplasty options to treat their specific pathology has allowed me to greatly expand my practice. I am very excited about what the MOTO system will become when the last puzzle piece, the MOTO PFJ, will be released early next year, allowing me to treat also the patellofemoral osteoarthritis. The system offers a comprehensive range of options aiming to intra-op flexibility and patients' well-being."
MOTO PFJ (Patello-Femoral Joint), with its anticipated availability in 2021, will allow for the treatment of patellofemoral osteoarthritis without compromising the non-affected tibiofemoral knee compartments. This implant features an onlay-anterior and inlay-distal design with a broad-anterior and narrow-distal shape, conceived to restore a painless patellar movement throughout the whole articular range of motion. The dedicated surgical technique and instrumentation guide every step of the procedure, utilizing the anterior cortex as reference and completely avoiding intramedullary canal violation or any free-hand preparation.
The introduction of these innovative implants and their cutting-edge concepts is possible through the M.O.R.E. Excellence Clinical Program, which defines the steps and milestones applicable to all Medacta products ahead of their full release. Under this program, Medacta releases new products on a restricted basis to conduct voluntary clinical programs in order to further document their safety and efficacy.
Discover more about Medacta's knee portfolio at knee.medacta.com.