Sam Brusco, Associate Editor01.28.21
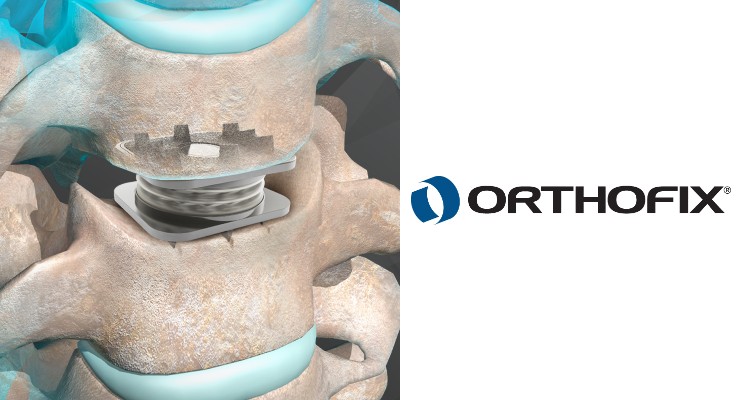
Orthofix published two-year data from its U.S. Investigational Device Exemption (IDE) study of the M6-C artificial cervical disc.
Published in The Spine Journal, results show treatment with the M6-C disc was safe, effective, and noninferior to anterior cervical discectomy and fusion (ACDF) for cervical disc degeneration. Designed to mimic a patient’s natural disc, the M6-C artificial cervical disc earned U.S. Food and Drug Administration (FDA) approval in 2019.
“The full two-year data demonstrates that patients with degenerative cervical radiculopathy treated with the M6-C disc had significant improvements in neck and arm pain, function and quality of life scores,” Dr. Frank Phillips, Professor of Orthopedic Surgery at Rush University Medical Center and a co-author of the journal article said in a press release. “Additionally, these patients had a significant difference in the reduction of pain and opioid medications use when compared to ACDF patients. At 24 months, patients in the ACDF group who were still using pain medications had a seven times higher rate of opioid use than those in the M6-C disc group.”
“The publication of the two-year data is important as it provides surgeons around the world the opportunity to see the full results of the M6-C artificial cervical disc study to further understand the benefits of this unique next-generation technology,” added Orthofix President of Global Spine Kevin Kenny. “We are proud to be able to provide this life-changing technology to surgeons and their patients. The M6-C disc is representative of our commitment to deliver innovative, quality-driven solutions that can improve patients’ lives.”
The M6-C IDE study was a prospective, non-randomized, concurrently controlled clinical trial that evaluated the M6-C artificial cervical disc's safety and effectiveness compared to ACDF for the treatment of single-level symptomatic cervical radiculopathy with or without cord compression.
Conducted at 23 sites in the U.S. with an average patient age of 44 years, the overall success rate for the protocol-specified primary endpoint for the M6-C disc patients was 86.8 percent at 24 months and 79.3 percent in the control group. This data statistically demonstrate that cervical disc replacement with the M6-C disc is not inferior to treatment with ACDF.
Secondary outcomes include:
Meaningful clinical improvement in the following pain scores:
Published in The Spine Journal, results show treatment with the M6-C disc was safe, effective, and noninferior to anterior cervical discectomy and fusion (ACDF) for cervical disc degeneration. Designed to mimic a patient’s natural disc, the M6-C artificial cervical disc earned U.S. Food and Drug Administration (FDA) approval in 2019.
“The full two-year data demonstrates that patients with degenerative cervical radiculopathy treated with the M6-C disc had significant improvements in neck and arm pain, function and quality of life scores,” Dr. Frank Phillips, Professor of Orthopedic Surgery at Rush University Medical Center and a co-author of the journal article said in a press release. “Additionally, these patients had a significant difference in the reduction of pain and opioid medications use when compared to ACDF patients. At 24 months, patients in the ACDF group who were still using pain medications had a seven times higher rate of opioid use than those in the M6-C disc group.”
“The publication of the two-year data is important as it provides surgeons around the world the opportunity to see the full results of the M6-C artificial cervical disc study to further understand the benefits of this unique next-generation technology,” added Orthofix President of Global Spine Kevin Kenny. “We are proud to be able to provide this life-changing technology to surgeons and their patients. The M6-C disc is representative of our commitment to deliver innovative, quality-driven solutions that can improve patients’ lives.”
The M6-C IDE study was a prospective, non-randomized, concurrently controlled clinical trial that evaluated the M6-C artificial cervical disc's safety and effectiveness compared to ACDF for the treatment of single-level symptomatic cervical radiculopathy with or without cord compression.
Conducted at 23 sites in the U.S. with an average patient age of 44 years, the overall success rate for the protocol-specified primary endpoint for the M6-C disc patients was 86.8 percent at 24 months and 79.3 percent in the control group. This data statistically demonstrate that cervical disc replacement with the M6-C disc is not inferior to treatment with ACDF.
Secondary outcomes include:
- Patients who received the M6-C disc demonstrated statistically significant improvement in the Neck Disability Index as measured at week six and months three, six, 12 and 24.
- Patients receiving the M6-C disc reported a 92-percent satisfaction rate with the surgery, and 93 percent said they would have the surgery again.
- Prior to surgery, 80.6 percent of the M6-C disc patients and 85.7 percent of the ACDF patients were taking some type of pain medication for the treatment of their cervical spine condition. At 24 months, the rate of M6-C patients who were still taking some type of pain medication dropped to 14.0 percent compared to 38.2 percent of the ACDF patients.
- Of these, there was a seven times higher rate of opioid use with the ACDF patients than with patients who received the M6-C disc.
Meaningful clinical improvement in the following pain scores:
- 91.2 percent of patients who received the M6-C disc reported an improvement in neck pain compared to 77.9 percent in patients who underwent the ACDF procedure.
- 90.5 percent of the M6-C patients reported improvement in arm pain scores compared to 79.9 percent in ACDF patients.
- There was a statistically significant difference in the average mean surgery time—74.5 minutes for patients receiving the M6-C disc versus 120.2 minutes for those patients having the ACDF procedure.
- In addition, there was a statistically significant difference in the mean length of hospital stay—0.61 days for the M6-C patients versus 1.10 days for ACDF patients.
- There were no device migrations reported in the study.
- Subsequent surgery at the treated level was needed in 1.9 percent of the M6-C disc patients compared to 4.8 percent of the ACDF patients
- There were 3.8 percent serious adverse events related to the device or procedure in the M6-C disc group versus 6.1 percent in the ACDF group.