Evolution Devices09.22.21
Evolution Devices has announced the launch of its EvoWalk Platform pilot program to rehabilitate walking for people living with neurologically-based partial walking paralysis. Evolution Devices’s novel approach combines remote neurologic physical therapy (PT) with the EvoWalk smart stimulation device to help patients avoid falls and reclaim instinctual movement, empowering them to walk more freely.
Evolution Devices is initially focused on rehabilitating Foot Drop, an impairment where a person is unable to lift their foot due to muscle weakness or nerve damage and which frequently causes falls. It generally results from stroke or Multiple Sclerosis (MS). The EvoWalk remote therapy platform allows these patients to meet with physical therapists (PTs) virtually, providing them with a personalized and comprehensive rehab program to help them improve their mobility.
“We are revolutionizing walking rehab by taking a holistic approach which combines virtual physical therapy with an AI-powered stimulation device to personalize care. For the millions of people who are living with mobility impairments, this combination is not currently available with any other fall-prevention or rehab therapy,” said Pierluigi Mantovani, co-founder and CEO of Evolution Devices.
Evolution Devices is currently conducting a pilot of the EvoWalk Platform through the company’s director of clinical services and neuro physical therapist, Lisa Donahue (N.C.S., M.P.T., P.T.) In addition, UCSF Physical Therapy is running an EvoWalk clinical study. Earlier pilot studies of the EvoWalk Platform revealed that patients experienced up to a tenfold increase in their walking activity and improved their walking speed in as little as eight weeks.
How the EvoWalk Platform Works:
The EvoWalk Device
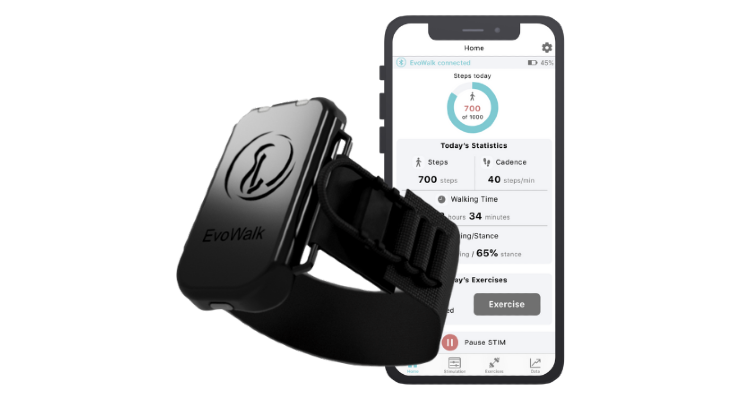
The EvoWalk device is worn around the leg just below the knee. It provides Functional Electrical Stimulation (FES) therapy to help patients to pick up their foot and walk more smoothly. By applying electrical stimulation to the lower leg, EvoWalk acts as an artificial nerve that bypasses the non-functioning nerve responsible for lifting the foot and toes.
The device’s built-in sensors feed real-time motion data to AI algorithms and provide actionable metrics through connected patient and clinician mobile apps, driving patient engagement. The patient app empowers patients by making it easy to monitor daily progress of key metrics, while the clinician app provides more detailed insights that enable PTs to remotely assess and refine their tailored rehab interventions.
Mantovani and his team of AI and neuroscience experts have been working on the device since 2017. Mantovani was inspired to build the EvoWalk to help his father who has progressing Multiple Sclerosis.
“My father’s MS caused him trouble with mobility as he struggled with Foot Drop. The EvoWalk Platform has significantly improved his walking, even as his MS continues to cause other challenges,” said Mantovani.
One early EvoWalk user, a hemiplegic stroke survivor with Foot Drop, finished a 5K in just over an hour, shattering his goal of 90 minutes for completing the race.
Evolution Devices has raised more than $1 million in funding from notable investors including the Alchemist Accelerator, as well as Edge Systems founder Bill Cohen who led the medical device company to a $150 million exit. In addition, the company has won grants from such organizations as the Toyota Mobility Foundation, Bristol Myers Squibb and Lyfebulb, the National Science Foundation, and the National Institutes of Health.
Evolution Devices recently launched an equity crowdfunding campaign to raise an additional $1 million which will enable them to accelerate commercialization efforts.
Evolution Devices is initially focused on rehabilitating Foot Drop, an impairment where a person is unable to lift their foot due to muscle weakness or nerve damage and which frequently causes falls. It generally results from stroke or Multiple Sclerosis (MS). The EvoWalk remote therapy platform allows these patients to meet with physical therapists (PTs) virtually, providing them with a personalized and comprehensive rehab program to help them improve their mobility.
“We are revolutionizing walking rehab by taking a holistic approach which combines virtual physical therapy with an AI-powered stimulation device to personalize care. For the millions of people who are living with mobility impairments, this combination is not currently available with any other fall-prevention or rehab therapy,” said Pierluigi Mantovani, co-founder and CEO of Evolution Devices.
Evolution Devices is currently conducting a pilot of the EvoWalk Platform through the company’s director of clinical services and neuro physical therapist, Lisa Donahue (N.C.S., M.P.T., P.T.) In addition, UCSF Physical Therapy is running an EvoWalk clinical study. Earlier pilot studies of the EvoWalk Platform revealed that patients experienced up to a tenfold increase in their walking activity and improved their walking speed in as little as eight weeks.
How the EvoWalk Platform Works:
- A patient is matched with a certified neurologic PT who assesses them remotely via a secure HIPAA-compliant video platform
- The patient receives the EvoWalk device and downloads the patient app
- Dedicated PTs train the patient on the EvoWalk and develop a customized therapy program which evolves as their rehab progresses
- While wearing the EvoWalk device, the patient walks more freely as it provides personalized stimulation that lifts the patient’s foot at precisely the right times
- Mobility data is automatically collected and shared with the patient and therapist and is used to update/refine their rehab therapy
The EvoWalk Device
The EvoWalk device is worn around the leg just below the knee. It provides Functional Electrical Stimulation (FES) therapy to help patients to pick up their foot and walk more smoothly. By applying electrical stimulation to the lower leg, EvoWalk acts as an artificial nerve that bypasses the non-functioning nerve responsible for lifting the foot and toes.
The device’s built-in sensors feed real-time motion data to AI algorithms and provide actionable metrics through connected patient and clinician mobile apps, driving patient engagement. The patient app empowers patients by making it easy to monitor daily progress of key metrics, while the clinician app provides more detailed insights that enable PTs to remotely assess and refine their tailored rehab interventions.
Mantovani and his team of AI and neuroscience experts have been working on the device since 2017. Mantovani was inspired to build the EvoWalk to help his father who has progressing Multiple Sclerosis.
“My father’s MS caused him trouble with mobility as he struggled with Foot Drop. The EvoWalk Platform has significantly improved his walking, even as his MS continues to cause other challenges,” said Mantovani.
One early EvoWalk user, a hemiplegic stroke survivor with Foot Drop, finished a 5K in just over an hour, shattering his goal of 90 minutes for completing the race.
Evolution Devices has raised more than $1 million in funding from notable investors including the Alchemist Accelerator, as well as Edge Systems founder Bill Cohen who led the medical device company to a $150 million exit. In addition, the company has won grants from such organizations as the Toyota Mobility Foundation, Bristol Myers Squibb and Lyfebulb, the National Science Foundation, and the National Institutes of Health.
Evolution Devices recently launched an equity crowdfunding campaign to raise an additional $1 million which will enable them to accelerate commercialization efforts.