Integrity Implants06.28.21
Integrity Implants Inc., a leader in Adaptive Geometry technology for spine surgery solutions, has received CE mark certification for its flagship FlareHawk Expandable Lumbar Interbody Fusion Device. FlareHawk launched in the United States in August 2016 and more than 10,500 FlareHawk devices have been implanted in more than 8,500 patients to date.
“CE mark further validates our belief that FlareHawk is the first expandable interbody solution that will have a true global reach,” said Chris Walsh, co-founder and CEO of Integrity Implants. “FlareHawk’s novel design and efficient manufacturing process enable access to the EU healthcare system. We are excited to be launching in several select European countries and initiating a prospective study with FlareHawk in the coming months. Our previously published data, which helped precipitate CE Mark clearance, is very strong data compared to that currently seen in both the expandable and TLIF/PLIF spaces.”
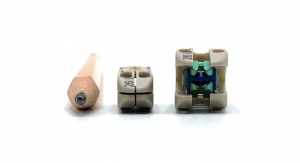
The uniqueness of the FlareHawk multidirectional interbody fusion cage called for a different approach than that used by many medical device manufacturers to demonstrate equivalence to other previously approved products. Instead, Integrity Implants compiled original retrospective clinical evidence data on FlareHawk’s performance and safety, including fusion rates, patient-reported outcome scores and adverse events.
“This data was pivotal in establishing evidence showing the safety and effectiveness of FlareHawk’s multidirectional expanding cage,” said the study’s principle investigator, Dom Coric, M.D., chief of Spine Division at Atrium Healthcare’s Musculoskeletal Institute and neurosurgeon at Carolina Neurosurgery & Spine Associates in Charlotte, N.C. “FlareHawk has provided exceptional outcomes for my patients, and I’m happy to have played a part in helping this innovative product receive approval for the benefit of many more patients throughout the European Union.”
The data utilized in the study followed strict inclusion criteria and was drawn from participants with comorbidities often seen in the general patient population, including high BMI, diabetes, coronary heart disease, hypertension and a history of smoking. The study also only allowed allograft or autograft to help facilitate fusion as opposed to more expensive spinal biologics like BMP, DBM or other processed biologics. Among the 129 study patients, TLIF (51 percent) or PLIF (49 percent) surgery was performed on 171 levels. A minimally invasive approach was used in 88 percent of the cases.
Outcomes were impressive, with 97.4 percent of levels achieving fusion based on the well-respected Bridwell-Lenke grading classification. Additionally, there were no reported device-related adverse events, no observations of cage subsidence (defined as an overlap between the vertebral endplates and the device exceeding 25 percent of the device height), and only one case (1.7 percent) of observed device migration (defined as displacement of the device relative to the position within intra-operative or immediate post-operative images). More than 70 percent of patients also demonstrated clinically significant improvements in VAS leg and back pain scores.
“Patient-reported outcomes, including ODI and VAS for leg and back pain, were significantly improved during the last patient follow-up, an extremely important benefit in the constant quest for optimal patient outcomes and satisfaction with value-based spine procedures,” said Raphael Roybal, M.D., M.B.A., director of The Spine Institute at Chatham Orthopaedics in Savannah, Ga., and co-author of the study. “The FlareHawk device has been transformational for my practice, especially during the past year, as it has allowed me to transition many cases to an outpatient setting that is safer for my patients and staff.”
Mark Grubb, M.D., a minimally invasive spine surgeon at Northeast Ohio Spine Center in Akron, Ohio and co-author of the paper, agreed: “FlareHawk’s small insertion profile and multidirectional expansion means I can treat the patient minimally invasively via a single position. The open architecture of the device also allows me to deliver the bone graft through the expanded FlareHawk cage and into the intervertebral space. This is a distinct improvement over other expandable products and represents another design aspect that I believe contributes to the favorable fusion rates seen with the FlareHawk device.”
In addition to receiving U.S. Food and Drug Administration clearance and CE mark approval, FlareHawk has also received approvals in Argentina, New Zealand, Taiwan and the United Arab Emirates.
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine.
“CE mark further validates our belief that FlareHawk is the first expandable interbody solution that will have a true global reach,” said Chris Walsh, co-founder and CEO of Integrity Implants. “FlareHawk’s novel design and efficient manufacturing process enable access to the EU healthcare system. We are excited to be launching in several select European countries and initiating a prospective study with FlareHawk in the coming months. Our previously published data, which helped precipitate CE Mark clearance, is very strong data compared to that currently seen in both the expandable and TLIF/PLIF spaces.”
The uniqueness of the FlareHawk multidirectional interbody fusion cage called for a different approach than that used by many medical device manufacturers to demonstrate equivalence to other previously approved products. Instead, Integrity Implants compiled original retrospective clinical evidence data on FlareHawk’s performance and safety, including fusion rates, patient-reported outcome scores and adverse events.
“This data was pivotal in establishing evidence showing the safety and effectiveness of FlareHawk’s multidirectional expanding cage,” said the study’s principle investigator, Dom Coric, M.D., chief of Spine Division at Atrium Healthcare’s Musculoskeletal Institute and neurosurgeon at Carolina Neurosurgery & Spine Associates in Charlotte, N.C. “FlareHawk has provided exceptional outcomes for my patients, and I’m happy to have played a part in helping this innovative product receive approval for the benefit of many more patients throughout the European Union.”
The data utilized in the study followed strict inclusion criteria and was drawn from participants with comorbidities often seen in the general patient population, including high BMI, diabetes, coronary heart disease, hypertension and a history of smoking. The study also only allowed allograft or autograft to help facilitate fusion as opposed to more expensive spinal biologics like BMP, DBM or other processed biologics. Among the 129 study patients, TLIF (51 percent) or PLIF (49 percent) surgery was performed on 171 levels. A minimally invasive approach was used in 88 percent of the cases.
Outcomes were impressive, with 97.4 percent of levels achieving fusion based on the well-respected Bridwell-Lenke grading classification. Additionally, there were no reported device-related adverse events, no observations of cage subsidence (defined as an overlap between the vertebral endplates and the device exceeding 25 percent of the device height), and only one case (1.7 percent) of observed device migration (defined as displacement of the device relative to the position within intra-operative or immediate post-operative images). More than 70 percent of patients also demonstrated clinically significant improvements in VAS leg and back pain scores.
“Patient-reported outcomes, including ODI and VAS for leg and back pain, were significantly improved during the last patient follow-up, an extremely important benefit in the constant quest for optimal patient outcomes and satisfaction with value-based spine procedures,” said Raphael Roybal, M.D., M.B.A., director of The Spine Institute at Chatham Orthopaedics in Savannah, Ga., and co-author of the study. “The FlareHawk device has been transformational for my practice, especially during the past year, as it has allowed me to transition many cases to an outpatient setting that is safer for my patients and staff.”
Mark Grubb, M.D., a minimally invasive spine surgeon at Northeast Ohio Spine Center in Akron, Ohio and co-author of the paper, agreed: “FlareHawk’s small insertion profile and multidirectional expansion means I can treat the patient minimally invasively via a single position. The open architecture of the device also allows me to deliver the bone graft through the expanded FlareHawk cage and into the intervertebral space. This is a distinct improvement over other expandable products and represents another design aspect that I believe contributes to the favorable fusion rates seen with the FlareHawk device.”
In addition to receiving U.S. Food and Drug Administration clearance and CE mark approval, FlareHawk has also received approvals in Argentina, New Zealand, Taiwan and the United Arab Emirates.
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine.