Globe Newswire09.21.21
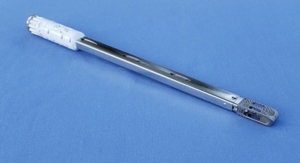
Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, has introduced the TiHawk9 expandable interbody cage, the latest addition to its FlareHawk family of spinal fusion cages, now featuring titanium at the bony interface.
“TiHawk represents an encouraging evolution in interbody cage design by integrating the desirable features of both PEEK and titanium,” said Sravisht Iyer, M.D., a spine surgeon specializing in minimally invasive surgery at Hospital for Special Surgery (HSS) in New York City. “TiHawk’s innovative application of titanium means I now have the surface characteristics I desire without the fear of surface delamination or undermining my ability to easily assess fusion through the cage after surgery.”
TiHawk features a uniform non-porous 0.5-micron-thick layer of titanium applied through a high-vacuum, low-temperature, ion beam-assisted deposition process. Unlike conventional physical vapor deposition coatings, the concurrent ion bombardment intermixes the titanium and PEEK atoms at the interface providing a strong physical bond and a resulting layer of titanium that provides the desired surface characteristics yet is thin enough not to impair radiographic visualization of implant placement and assessment of fusion. Standard titanium-coated implants often generate a ghost artifact under fluoroscopic imaging, which require patients to get costly, time-consuming and high radiation-generating CT scans to confirm fusion.
“I have used the FlareHawk cage with great success and welcome the titanium-bonded version as a treatment option for my patients,” said Lee Tan, M.D., assistant professor of Neurological Surgery at UCSF Medical Center in San Francisco, who performed the first TiHawk case. “In addition to providing radial expansion and endplate conformability from its multimaterial construct, TiHawk features titanium at the bony interface. It also improves the radiographic visibility of the cage shell which helps surgeons to better assess the cage position intraoperatively and post-operatively.”
The first-of-its-kind FlareHawk family of implants utilize a PEEK shell and internal shim, providing a small insertion profile to minimize neural retraction during insertion and then allowing expansion in height, width and lordosis to potentially reduce subsidence, restore foraminal height and re-establish sagittal balance. Peer-reviewed clinical studies have demonstrated FlareHawk’s adaptive geometry as a potential contributor to its positive fusion rates, as well as to the absence of observed subsidence1-3–a concern with monolithic titanium cages that present a mismatch in the modulus of elasticity between the implant and the bone. To date, over 10,500 FlareHawk cages have been implanted in more than 9,000 patients.
“Surgeons loved what they were seeing from our PEEK FlareHawk device and its ability to conform to the vertebral endplates; however, they also desired a titanium option since titanium is recognized for potentially enhancing bone fixation,” said Chris Walsh, co-founder and CEO of Accelus. “We are thrilled with the final TiHawk device, as it features a layer of titanium that still allows for a surgeon to assess fusion radiographically in the office, as well as the same minimal insertion profile, multidirectional expansion, and maximum bone graft delivery that surgeons have come to expect from FlareHawk interbody devices.”
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine.
Accelus is committed to accelerating minimally invasive spine surgery through its enabling technology with broad accessibility to previously underserved markets. Established in 2021 through the combination of Integrity Implants and Fusion Robotics, the company is focused on providing its proprietary Adaptive Geometry technology with pragmatic and economical navigation and robotic solutions with broad clinical use in spine surgery.
References
1 Tan LA, Rivera J, Tan XA, Le VP, Khoo LT, Berven SH. Clinical and Radiographic Outcomes After Minimally Invasive Transforaminal Lumbar Interbody Fusion-Early Experience Using a Biplanar Expandable Cage for Lumbar Spondylolisthesis. Int J Spine Surg. 2020 Dec;14(s3):S39-S44. doi: 10.14444/7125. Epub 2020 Oct 29. PMID: 33122185; PMCID: PMC7735467.
2 Coric D, Roybal RR, Grubb M, Rossi V, Yu AK, Swink IR, Long J, Cheng BC, Inzana JA. Bidirectional Expandable Technology for Transforaminal or Posterior Lumbar Interbody Fusion: A Retrospective Analysis of Safety and Performance. Int J Spine Surg. 2020 Dec;14(s3):S22-S30. doi: 10.14444/7123. Epub 2020 Oct 29. PMID: 33122186; PMCID: PMC7735440.
3 Cheng BC, Swink I, Yusufbekov R, Birgelen Michele, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S68. Published December 1, 2020.
“TiHawk represents an encouraging evolution in interbody cage design by integrating the desirable features of both PEEK and titanium,” said Sravisht Iyer, M.D., a spine surgeon specializing in minimally invasive surgery at Hospital for Special Surgery (HSS) in New York City. “TiHawk’s innovative application of titanium means I now have the surface characteristics I desire without the fear of surface delamination or undermining my ability to easily assess fusion through the cage after surgery.”
TiHawk features a uniform non-porous 0.5-micron-thick layer of titanium applied through a high-vacuum, low-temperature, ion beam-assisted deposition process. Unlike conventional physical vapor deposition coatings, the concurrent ion bombardment intermixes the titanium and PEEK atoms at the interface providing a strong physical bond and a resulting layer of titanium that provides the desired surface characteristics yet is thin enough not to impair radiographic visualization of implant placement and assessment of fusion. Standard titanium-coated implants often generate a ghost artifact under fluoroscopic imaging, which require patients to get costly, time-consuming and high radiation-generating CT scans to confirm fusion.
“I have used the FlareHawk cage with great success and welcome the titanium-bonded version as a treatment option for my patients,” said Lee Tan, M.D., assistant professor of Neurological Surgery at UCSF Medical Center in San Francisco, who performed the first TiHawk case. “In addition to providing radial expansion and endplate conformability from its multimaterial construct, TiHawk features titanium at the bony interface. It also improves the radiographic visibility of the cage shell which helps surgeons to better assess the cage position intraoperatively and post-operatively.”
The first-of-its-kind FlareHawk family of implants utilize a PEEK shell and internal shim, providing a small insertion profile to minimize neural retraction during insertion and then allowing expansion in height, width and lordosis to potentially reduce subsidence, restore foraminal height and re-establish sagittal balance. Peer-reviewed clinical studies have demonstrated FlareHawk’s adaptive geometry as a potential contributor to its positive fusion rates, as well as to the absence of observed subsidence1-3–a concern with monolithic titanium cages that present a mismatch in the modulus of elasticity between the implant and the bone. To date, over 10,500 FlareHawk cages have been implanted in more than 9,000 patients.
“Surgeons loved what they were seeing from our PEEK FlareHawk device and its ability to conform to the vertebral endplates; however, they also desired a titanium option since titanium is recognized for potentially enhancing bone fixation,” said Chris Walsh, co-founder and CEO of Accelus. “We are thrilled with the final TiHawk device, as it features a layer of titanium that still allows for a surgeon to assess fusion radiographically in the office, as well as the same minimal insertion profile, multidirectional expansion, and maximum bone graft delivery that surgeons have come to expect from FlareHawk interbody devices.”
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine.
Accelus is committed to accelerating minimally invasive spine surgery through its enabling technology with broad accessibility to previously underserved markets. Established in 2021 through the combination of Integrity Implants and Fusion Robotics, the company is focused on providing its proprietary Adaptive Geometry technology with pragmatic and economical navigation and robotic solutions with broad clinical use in spine surgery.
References
1 Tan LA, Rivera J, Tan XA, Le VP, Khoo LT, Berven SH. Clinical and Radiographic Outcomes After Minimally Invasive Transforaminal Lumbar Interbody Fusion-Early Experience Using a Biplanar Expandable Cage for Lumbar Spondylolisthesis. Int J Spine Surg. 2020 Dec;14(s3):S39-S44. doi: 10.14444/7125. Epub 2020 Oct 29. PMID: 33122185; PMCID: PMC7735467.
2 Coric D, Roybal RR, Grubb M, Rossi V, Yu AK, Swink IR, Long J, Cheng BC, Inzana JA. Bidirectional Expandable Technology for Transforaminal or Posterior Lumbar Interbody Fusion: A Retrospective Analysis of Safety and Performance. Int J Spine Surg. 2020 Dec;14(s3):S22-S30. doi: 10.14444/7123. Epub 2020 Oct 29. PMID: 33122186; PMCID: PMC7735440.
3 Cheng BC, Swink I, Yusufbekov R, Birgelen Michele, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S68. Published December 1, 2020.