Ravi Kiran, Lead Consultant, The Business Research Company04.03.18
The global market for minor orthopedic replacement implants exceeded $1.5 billion in 2017. Analysts from The Business Research Company forecast the market value to grow at a rate of over 7 percent and reach $2.2 billion in 2021, in their report titled Minor Orthopedic Replacement Implants. According to the report, the reason for this growth is a result of the high incidence of arthritis and fractures. Osteoarthritis and rheumatoid arthritis are the major causes for joint replacement surgery including minor orthopedic joints. The other cause for joint replacement surgery is failed previous joint replacement surgery.
Segments of the Market
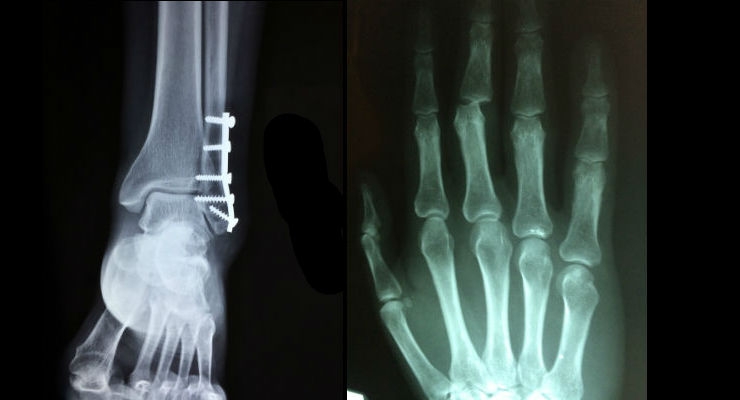
Minor orthopedic implants include implants used in shoulder, wrist, ankle, and foot joints. Shoulder replacement holds the largest market share, capturing the majority of the market. Growth in all four segments—shoulder, wrist, elbow, and foot & ankle implants—has accelerated recently.
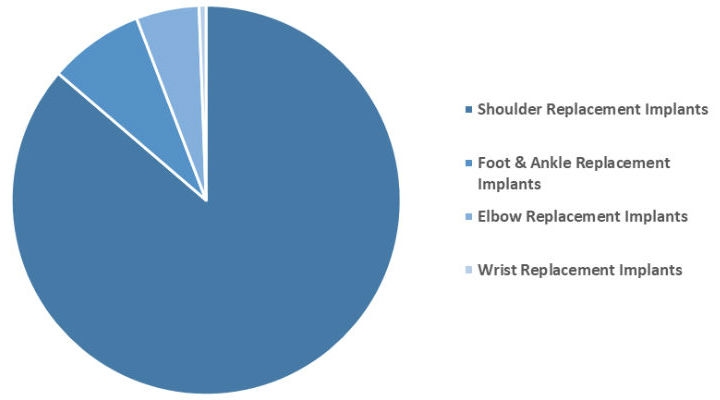
Chart: Global Minor Orthopedic Replacement Implants Market, 2017 (split by segments). Chart courtesy of The Business Research Company.
Regional Analysis
With more than three-fifths of the market share, North America was the largest region in the minor orthopedic replacement implants market in 2017. The United States was the largest country in the market, accounting for more than half the market share. The smallest market for minor orthopedic implants was in South America with a market share of over 1 percent.
Better Materials Driving the Market
Minor joint replacements are highly complex and have previously suffered from a high failure rate. There have been technological advances in both the implants themselves and the surgeries involved, which have contributed to the growth of the market. For example, metals such as titanium, cobalt, chromium, molybdenum, and other new materials such as polyethylene have replaced the plastics and ceramics that were earlier being used in the design and manufacture of implants. The metals have a porous surface that enhances osteointegration, thus reducing revision rates. Polyethylene reduces wear and improves the longevity of the minor joint replacement implants. This shift toward new, high quality materials in implants has driven the market since 2013. Longer life for implants makes them suitable for use in younger fracture patients, for whom they were previously not recommended owing to the need for replacement every 10 to 15 years.
Generic Orthopedic Implant Companies Gain Traction
Following the expiry of patents for implants, around sixteen generic companies have entered the orthopedic replacement implants market in the U.S., including Orthosolutions, Covenant Orthopedics, Ortho Direct USA, and Emerge Medical. These generic companies are making copies of legacy implants with proven designs and biomaterials. These generic orthopedic implant companies offer lower prices compared to branded implants and, thus, are a significant threat for the larger orthopedic devices manufacturers such as Stryker, J&J/DePuy/Synthes, Zimmer Biomet, Smith & Nephew, and Medtronic Spine.
As there is wide scope of entry for many generic orthopedic device manufacturing companies, these generic companies should focus on marketing strategies in order to compete with the more dominant orthopedic manufacturers.
Interest of Major Orthopedic Implant Manufacturers in Emerging Markets
The BRIC countries have faster growing economies than the U.S., and represent around 41 percent of total world population. Due to converging uncertainty about regulatory, economic, and reimbursement trends in the U.S. market, the aforementioned “major players” are shifting their resources, training, inventories, clinical studies, and R&D efforts to these four emerging orthopedic markets.
Due to high growth for orthopedic implants in BRIC countries, other orthopedic implant manufacturers (beyond the “big 5”) should also enter the market to increase profits.
Positive Trend for Minor Joint Procedures
Recently, small bone plating, especially in the distal radius segment, has been a major trend. There is also an increasing trend toward new shoulder prostheses and total ankle replacements. For example, 100,000 shoulder arthroplasties were performed in 2016 in the U.S., compared to only 14,000 in 2000. This rapid rate of growth is largely due to the expanding indications for minor joint procedures and it’s rising popularity, and the size of the aging population that desires to remain active.
Increased Use of Reverse Shoulder Arthroplasty
The procedure numbers for reverse shoulder arthroplasty are continuously increasing. According to The Business Research Company’s report on minor orthopedic replacement implants, approximately half of the shoulder replacements in the U.S., and over two-thirds of shoulder replacements in Europe are reverse shoulder replacement surgeries. The balance of shoulder replacements are total shoulder arthroplasty or hemi-arthroplasty. This increased trend toward reverse shoulder arthroplasties are due to their increasing utilization to treat shoulder fracture patients, expanding applications to both elderly and more active patients, the highly successful outcomes of the procedure, and surgeons becoming more comfortable with the procedure.
Regulation Dominates the Minor Orthopedic Market
The medical devices market is highly regulated with authorities and regulatory bodies governing the development and marketing activities of medical device manufacturers in each country. The medical devices have to comply with quality standards developed by international organization for standardization (ISO).
Due to the financial pressures on healthcare budgets in many European countries, several governments are trying to reduce the cost of healthcare devices and improve their efficiency. Increasing privatization of healthcare is also forcing manufacturers to reduce the product costs. In some European countries, several healthcare segments such as orthopedics, plastic and corrective surgery, aesthetic surgery, and total pain management are being privatized.
Regulatory Scenario in U.S.
In the U.S., medical device manufacturers must submit a premarket notification to introduce a device for commercial distribution for the first time or reintroduce a device that will be significantly changed or modified to the extent that its safety or effectiveness could be affected. The changes may be related to the design, material, and chemical composition, energy source, manufacturing process, or intended use.
In terms of the price of the implants, the U.S. federal government, for instance, is unwilling to intervene directly to reduce the cost of joint replacement implants. Due to this, the insurance companies are pressuring implant manufacturers to reduce their prices.
In contrast to the situation in the U.S., the Indian government has capped the prices for orthopedic implants, which regulates the margin for these implant companies.

Segments of the Market
Minor orthopedic implants include implants used in shoulder, wrist, ankle, and foot joints. Shoulder replacement holds the largest market share, capturing the majority of the market. Growth in all four segments—shoulder, wrist, elbow, and foot & ankle implants—has accelerated recently.

Chart: Global Minor Orthopedic Replacement Implants Market, 2017 (split by segments). Chart courtesy of The Business Research Company.
Regional Analysis
With more than three-fifths of the market share, North America was the largest region in the minor orthopedic replacement implants market in 2017. The United States was the largest country in the market, accounting for more than half the market share. The smallest market for minor orthopedic implants was in South America with a market share of over 1 percent.
Better Materials Driving the Market
Minor joint replacements are highly complex and have previously suffered from a high failure rate. There have been technological advances in both the implants themselves and the surgeries involved, which have contributed to the growth of the market. For example, metals such as titanium, cobalt, chromium, molybdenum, and other new materials such as polyethylene have replaced the plastics and ceramics that were earlier being used in the design and manufacture of implants. The metals have a porous surface that enhances osteointegration, thus reducing revision rates. Polyethylene reduces wear and improves the longevity of the minor joint replacement implants. This shift toward new, high quality materials in implants has driven the market since 2013. Longer life for implants makes them suitable for use in younger fracture patients, for whom they were previously not recommended owing to the need for replacement every 10 to 15 years.
Generic Orthopedic Implant Companies Gain Traction
Following the expiry of patents for implants, around sixteen generic companies have entered the orthopedic replacement implants market in the U.S., including Orthosolutions, Covenant Orthopedics, Ortho Direct USA, and Emerge Medical. These generic companies are making copies of legacy implants with proven designs and biomaterials. These generic orthopedic implant companies offer lower prices compared to branded implants and, thus, are a significant threat for the larger orthopedic devices manufacturers such as Stryker, J&J/DePuy/Synthes, Zimmer Biomet, Smith & Nephew, and Medtronic Spine.
As there is wide scope of entry for many generic orthopedic device manufacturing companies, these generic companies should focus on marketing strategies in order to compete with the more dominant orthopedic manufacturers.
Interest of Major Orthopedic Implant Manufacturers in Emerging Markets
The BRIC countries have faster growing economies than the U.S., and represent around 41 percent of total world population. Due to converging uncertainty about regulatory, economic, and reimbursement trends in the U.S. market, the aforementioned “major players” are shifting their resources, training, inventories, clinical studies, and R&D efforts to these four emerging orthopedic markets.
Due to high growth for orthopedic implants in BRIC countries, other orthopedic implant manufacturers (beyond the “big 5”) should also enter the market to increase profits.
Positive Trend for Minor Joint Procedures
Recently, small bone plating, especially in the distal radius segment, has been a major trend. There is also an increasing trend toward new shoulder prostheses and total ankle replacements. For example, 100,000 shoulder arthroplasties were performed in 2016 in the U.S., compared to only 14,000 in 2000. This rapid rate of growth is largely due to the expanding indications for minor joint procedures and it’s rising popularity, and the size of the aging population that desires to remain active.
Increased Use of Reverse Shoulder Arthroplasty
The procedure numbers for reverse shoulder arthroplasty are continuously increasing. According to The Business Research Company’s report on minor orthopedic replacement implants, approximately half of the shoulder replacements in the U.S., and over two-thirds of shoulder replacements in Europe are reverse shoulder replacement surgeries. The balance of shoulder replacements are total shoulder arthroplasty or hemi-arthroplasty. This increased trend toward reverse shoulder arthroplasties are due to their increasing utilization to treat shoulder fracture patients, expanding applications to both elderly and more active patients, the highly successful outcomes of the procedure, and surgeons becoming more comfortable with the procedure.
Regulation Dominates the Minor Orthopedic Market
The medical devices market is highly regulated with authorities and regulatory bodies governing the development and marketing activities of medical device manufacturers in each country. The medical devices have to comply with quality standards developed by international organization for standardization (ISO).
Due to the financial pressures on healthcare budgets in many European countries, several governments are trying to reduce the cost of healthcare devices and improve their efficiency. Increasing privatization of healthcare is also forcing manufacturers to reduce the product costs. In some European countries, several healthcare segments such as orthopedics, plastic and corrective surgery, aesthetic surgery, and total pain management are being privatized.
Regulatory Scenario in U.S.
In the U.S., medical device manufacturers must submit a premarket notification to introduce a device for commercial distribution for the first time or reintroduce a device that will be significantly changed or modified to the extent that its safety or effectiveness could be affected. The changes may be related to the design, material, and chemical composition, energy source, manufacturing process, or intended use.
In terms of the price of the implants, the U.S. federal government, for instance, is unwilling to intervene directly to reduce the cost of joint replacement implants. Due to this, the insurance companies are pressuring implant manufacturers to reduce their prices.
In contrast to the situation in the U.S., the Indian government has capped the prices for orthopedic implants, which regulates the margin for these implant companies.