Michael Barbella, Managing Editor03.18.19
Christopher J. Centeno, M.D., could arguably be considered the “father” of regenerative medicine. He claims, after all, to be the first person on the planet to perform stem cell injections to treat joint arthritis and various other conditions.
There are several worthy contenders, including Arnie Caplan, Ph.D., pioneer of mesenchymal stem cell use (late 1980s); Philippe Henigou, developer of bone marrow concentrate treatment for bone disease (early 1990s); or Svante Gehring, M.D., the first physician to document use of platelet-rich plasma in ophthalmology (1999).
Centeno, however, believes the title should go to Ohio surgeon George S. Hackett, M.D., who clinically and scientifically demonstrated the efficacy of irritating tissue to promote healing back in 1963. Hackett’s studies showed that creating controlled inflammation could increase ligament size by up to 35-40 percent (more recent analyses have confirmed those initial findings).
While certainly revolutionary, Hackett’s work nevertheless was pre-dated by several thousand years: The concept of irritating tissue to promote healing actually originated with the ancient Greeks. Hippocrates reportedly treated Olympic javelin throwers with unstable shoulders by touching a “slender hot iron” to the joint’s ligaments (the heat would irritate the ligament, causing it to tighten).
Chronologically speaking, then, Hippocrates is deserving of the regenerative medicine “father” title. Yet Centeno maintains that Hackett warrants a place in history, if only for his contributions to the evolution of the field.
“So, is there one modern ‘father’?” Centeno, an international expert and regenerative medicine expert, asked in a blog last spring. “You could make arguments for Hernigou, Caplan, the early users of PRP, and so on. However, if you had to award the title to one person, that honor likely goes to George Hackett, who figured out that you could help ligaments heal with injections.”
Regardless of its origins, regenerative medicine has become a fast-growing subsegment of the multi billion-dollar orthobiologics industry, thanks to an aging world population and advanced stem cell/gene therapy technologies in developed countries. ODT’s January/February feature “Natural Selection” examines the innovation being developed in this burgeoning sector that eventually will contribute to its double-digit annual growth over the next half-decade.
Clay Shors, Ph.D., president of Irvine, Calif.-based Biogennix, shared his insights on the market for the story. His full input is provided in the following Q&A.
Michael Barbella: Please discuss the current trends you see shaping the orthobiologics market.
Clay Shors: There are a number of trends that are clearly making an impact in orthobiologics today. First, there is an increased awareness and attention on synthetic osteoconductive scaffold innovations. There is growing demand for synthetic bone grafting substitutes that provide better handling properties for physicians, aiding in ease-of-use and making bone grafting more efficient. Bone graft substitutes that are biocompatible, resorbable, and aid in enhancing efficiency and results are sought after right now.
In addition, stem cells and stem cell-related technologies are popular particularly for the patient. This is driving some orthobiologic companies to focus on and innovate completmentary technologies. Wev'e also seen a significant rise in the number of procedures that are taking place in Ambulatory Surgery Centers (ASCs) as an alternative to hospital in-patient procedures. This is a factor that is also making the use of innovative synthetic scaffolds more appealing and cost-effective.
Barbella: What factors are driving innovation in the orthobiologics market?
Shors: There are several elements that have been playing key roles in pushing for increased innovation in the orthobiologics space. One factor is the shifting demand from mechanical to biological solutions, which has been a meaningful trend. Another is demographics. We have a rapidly aging population with an improved life expectancy compared to generations past. Seventy is the new 50, and patients are living longer, more active, and want to live a pain-free life. All of these factors lead to new and evolving needs for an older but healthier population.
In addition, with more people in the work environment sitting for prolonged periods of time, back pain is a lifestyle-affecting condition that has become more and more pervasive than ever before. Addictive pain medications such as OxyContin are now being less widely administered due to the current opioid addiction crisis. What we're seeing as a result is a distinct upward trend of patients opting for surgery, thereby driving further innovation.
Barbella: What factors are impeding growth in the orthobiologics market? How can these challenges be overcome?
Shors: One hurdle impeding growth in the orthobiologics market is the unpredictable and evolving hospital reimbursement scenario. This, and increased hospital consolidation, are current growth challenges for many orthobiologics companies today. Another obstacle that we're facing is the high costs of conducting clinical trials and the types of tests required. To this point, the increasing resources necessary for an organization in this space to achieve FDA approval in order to make certain claims can, in some cases, be cost-prohibitive.
Although challenges exist, we believe sound science and thoughtful innovation find a way. It might just unfortunately take longer for certain solutions to come to market than consumers demand due to the hurdles discussed above.
Barbella: What new technologies are in the works?
Shors: At Biogennix, we're excited about the immediate future and have a healthy product roadmap, with several new products expected to hit hospital shelves within the 2019 calendar year. To note, we are currently planning on launching two new osteoconductive bone graft solutions before the end of 2019. Additionally, we are in the process of conducting necessary development and testing and are marching towards a commercial launch on both products by Q4 2019. These two products will further enhance our product offering and will offer the clinician additional alternatives to our existing portfolio.
Barbella: Where does innovation come from in the orthobiologics market? How does Biogennix stay innovative amid all the competition?
Shors: At Biogennix, innovation initially comes from feedback we receive from key clinicians, as well as current trends shaping not only our industry but also other areas within medicine. Amid all the competition, Biogennix can stay innovative and relevant by staying close to surgeons and distributors, understanding their needs, and staying on top of current trends inside and outside the industry. Also important is a continued focus on building and strengthening relationships with key hospital systems, group purchasing organizations, and distribution partners.
Barbella: How does the regulatory landscape for orthobiologics differ from other sectors of the orthopedic market, and how does this affect product innovation?
Shors: The regulatory landscape may differ somewhat for orthobiologics in terms of the higher level of scrutiny placed on new materials, technologies, and applications. For example, the distinction for orthobiologics versus many structural devices in bone reconstruction is that the regulatory approvals require demonstration of efficacy based on animal models, whereas approval of most orthopedic structural devices is typically based solely on in-vitro mechanical testing. Animal testing is generally much more expensive, has variable results, and is more time-consuming.
Of course, with innovation comes the task of assessing and addressing new risks, which can slow down product innovation. For more agile orthobiologics companies such as Biogennix, with our solid team of product research and development experts and industry veterans, we're confident we will continue to deliver innovative products to the market.
Barbella: What is the dominant orthobiologics application—spinal fusion, trauma repair, reconstructive surgery? What factors are driving growth in this particular application?
Shors: Due to the increased average age of the population, as well as the lifestyle factors touched on above, the rate of spinal fusion procedures has risen rapidly over the past several decades. In addition, as structural and biologic reconstructive solutions continue to be improved and demonstrate procedural success, more patients seek these treatments, more surgeons dedicate their practices to these treatments, further contributing to the growth cycle.
With close to half a million spinal fusion procedures performed each year and growing, spinal fusion appears to be the dominating orthobiologics application. Of course, at Biogennix, we are targeting spine, which is the largest segment. However, it is worth mentioning that there are several additional growing niche segments such as long-bone fractures, bone tumors, joint (hip and knee) revision surgeries with bone loss, small bone (foot and hand), oral-maxillofacial/plastic surgery, periodontal, and veterinary, which all require innovation when it comes to orthobiologics products.
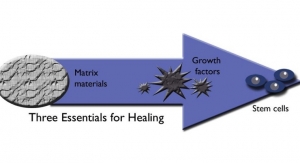
Lastly, with spinal fusion surgery, the drive for growth comes from an increased understanding by surgeons, patients, and the industry that fusion comes from bone regeneration. There is a growing acceptance that bone requires osteoconduction, osteogenesis, and osteoinduction. Successful regeneration comes from an equal combination of each of these factors. Reconstruction, in general, is complex and is not a one-size-fits-all science. The therapeutic constructs available for one patient might not be the best option for another. This demand for options lends itself to further innovation and orthobiologics solutions.
There are several worthy contenders, including Arnie Caplan, Ph.D., pioneer of mesenchymal stem cell use (late 1980s); Philippe Henigou, developer of bone marrow concentrate treatment for bone disease (early 1990s); or Svante Gehring, M.D., the first physician to document use of platelet-rich plasma in ophthalmology (1999).
Centeno, however, believes the title should go to Ohio surgeon George S. Hackett, M.D., who clinically and scientifically demonstrated the efficacy of irritating tissue to promote healing back in 1963. Hackett’s studies showed that creating controlled inflammation could increase ligament size by up to 35-40 percent (more recent analyses have confirmed those initial findings).
While certainly revolutionary, Hackett’s work nevertheless was pre-dated by several thousand years: The concept of irritating tissue to promote healing actually originated with the ancient Greeks. Hippocrates reportedly treated Olympic javelin throwers with unstable shoulders by touching a “slender hot iron” to the joint’s ligaments (the heat would irritate the ligament, causing it to tighten).
Chronologically speaking, then, Hippocrates is deserving of the regenerative medicine “father” title. Yet Centeno maintains that Hackett warrants a place in history, if only for his contributions to the evolution of the field.
“So, is there one modern ‘father’?” Centeno, an international expert and regenerative medicine expert, asked in a blog last spring. “You could make arguments for Hernigou, Caplan, the early users of PRP, and so on. However, if you had to award the title to one person, that honor likely goes to George Hackett, who figured out that you could help ligaments heal with injections.”
Regardless of its origins, regenerative medicine has become a fast-growing subsegment of the multi billion-dollar orthobiologics industry, thanks to an aging world population and advanced stem cell/gene therapy technologies in developed countries. ODT’s January/February feature “Natural Selection” examines the innovation being developed in this burgeoning sector that eventually will contribute to its double-digit annual growth over the next half-decade.
Clay Shors, Ph.D., president of Irvine, Calif.-based Biogennix, shared his insights on the market for the story. His full input is provided in the following Q&A.
Michael Barbella: Please discuss the current trends you see shaping the orthobiologics market.
Clay Shors: There are a number of trends that are clearly making an impact in orthobiologics today. First, there is an increased awareness and attention on synthetic osteoconductive scaffold innovations. There is growing demand for synthetic bone grafting substitutes that provide better handling properties for physicians, aiding in ease-of-use and making bone grafting more efficient. Bone graft substitutes that are biocompatible, resorbable, and aid in enhancing efficiency and results are sought after right now.
In addition, stem cells and stem cell-related technologies are popular particularly for the patient. This is driving some orthobiologic companies to focus on and innovate completmentary technologies. Wev'e also seen a significant rise in the number of procedures that are taking place in Ambulatory Surgery Centers (ASCs) as an alternative to hospital in-patient procedures. This is a factor that is also making the use of innovative synthetic scaffolds more appealing and cost-effective.
Barbella: What factors are driving innovation in the orthobiologics market?
Shors: There are several elements that have been playing key roles in pushing for increased innovation in the orthobiologics space. One factor is the shifting demand from mechanical to biological solutions, which has been a meaningful trend. Another is demographics. We have a rapidly aging population with an improved life expectancy compared to generations past. Seventy is the new 50, and patients are living longer, more active, and want to live a pain-free life. All of these factors lead to new and evolving needs for an older but healthier population.
In addition, with more people in the work environment sitting for prolonged periods of time, back pain is a lifestyle-affecting condition that has become more and more pervasive than ever before. Addictive pain medications such as OxyContin are now being less widely administered due to the current opioid addiction crisis. What we're seeing as a result is a distinct upward trend of patients opting for surgery, thereby driving further innovation.
Barbella: What factors are impeding growth in the orthobiologics market? How can these challenges be overcome?
Shors: One hurdle impeding growth in the orthobiologics market is the unpredictable and evolving hospital reimbursement scenario. This, and increased hospital consolidation, are current growth challenges for many orthobiologics companies today. Another obstacle that we're facing is the high costs of conducting clinical trials and the types of tests required. To this point, the increasing resources necessary for an organization in this space to achieve FDA approval in order to make certain claims can, in some cases, be cost-prohibitive.
Although challenges exist, we believe sound science and thoughtful innovation find a way. It might just unfortunately take longer for certain solutions to come to market than consumers demand due to the hurdles discussed above.
Barbella: What new technologies are in the works?
Shors: At Biogennix, we're excited about the immediate future and have a healthy product roadmap, with several new products expected to hit hospital shelves within the 2019 calendar year. To note, we are currently planning on launching two new osteoconductive bone graft solutions before the end of 2019. Additionally, we are in the process of conducting necessary development and testing and are marching towards a commercial launch on both products by Q4 2019. These two products will further enhance our product offering and will offer the clinician additional alternatives to our existing portfolio.
Barbella: Where does innovation come from in the orthobiologics market? How does Biogennix stay innovative amid all the competition?
Shors: At Biogennix, innovation initially comes from feedback we receive from key clinicians, as well as current trends shaping not only our industry but also other areas within medicine. Amid all the competition, Biogennix can stay innovative and relevant by staying close to surgeons and distributors, understanding their needs, and staying on top of current trends inside and outside the industry. Also important is a continued focus on building and strengthening relationships with key hospital systems, group purchasing organizations, and distribution partners.
Barbella: How does the regulatory landscape for orthobiologics differ from other sectors of the orthopedic market, and how does this affect product innovation?
Shors: The regulatory landscape may differ somewhat for orthobiologics in terms of the higher level of scrutiny placed on new materials, technologies, and applications. For example, the distinction for orthobiologics versus many structural devices in bone reconstruction is that the regulatory approvals require demonstration of efficacy based on animal models, whereas approval of most orthopedic structural devices is typically based solely on in-vitro mechanical testing. Animal testing is generally much more expensive, has variable results, and is more time-consuming.
Of course, with innovation comes the task of assessing and addressing new risks, which can slow down product innovation. For more agile orthobiologics companies such as Biogennix, with our solid team of product research and development experts and industry veterans, we're confident we will continue to deliver innovative products to the market.
Barbella: What is the dominant orthobiologics application—spinal fusion, trauma repair, reconstructive surgery? What factors are driving growth in this particular application?
Shors: Due to the increased average age of the population, as well as the lifestyle factors touched on above, the rate of spinal fusion procedures has risen rapidly over the past several decades. In addition, as structural and biologic reconstructive solutions continue to be improved and demonstrate procedural success, more patients seek these treatments, more surgeons dedicate their practices to these treatments, further contributing to the growth cycle.
With close to half a million spinal fusion procedures performed each year and growing, spinal fusion appears to be the dominating orthobiologics application. Of course, at Biogennix, we are targeting spine, which is the largest segment. However, it is worth mentioning that there are several additional growing niche segments such as long-bone fractures, bone tumors, joint (hip and knee) revision surgeries with bone loss, small bone (foot and hand), oral-maxillofacial/plastic surgery, periodontal, and veterinary, which all require innovation when it comes to orthobiologics products.
Lastly, with spinal fusion surgery, the drive for growth comes from an increased understanding by surgeons, patients, and the industry that fusion comes from bone regeneration. There is a growing acceptance that bone requires osteoconduction, osteogenesis, and osteoinduction. Successful regeneration comes from an equal combination of each of these factors. Reconstruction, in general, is complex and is not a one-size-fits-all science. The therapeutic constructs available for one patient might not be the best option for another. This demand for options lends itself to further innovation and orthobiologics solutions.