Marc Knebel, Global Segment Head Medical Systems—Head of VESTAKEEP Europe bei Evonik Operations GmbH02.11.21
Dogs are faithful companions. The four-legged darlings keep families fit, serve as life friends, and provide comfort. They also suffer when their health is weakened, such as in the case of hip dysplasia. To relieve dogs and even cats from pain and to regain their natural agility, the Swiss company KYON markets an innovative hip prosthesis based on the high-performance polymer VESTAKEEP PEEK from Evonik. Collaborating with potential medical device manufacturers, the specialty chemicals company now wants to transfer its material expertise and application knowledge from veterinary use to human medicine.
Whether internal fixation of bone fractures, treatment of cruciate rupture, or total hip replacement, many of today's veterinary treatment methods would not be conceivable at all without the underlying highly regulated human medicine. As a rule, veterinary medicine participates in the achievements of medical devices approved for humans by the FDA (U.S. Food and Drug Administration) or the PMDA (Japan’s Pharmaceuticals and Medical Devices Agency). The polymer experts from the specialty chemicals company Evonik wanted to see it happen the other way around. According to their idea, high-performance, proven materials used in the treatment of hip dysplasia in dogs and cats should be used in human medicine. The success of the innovative technology so far could prove them right.
Innovative Hip Prostheses for Pets
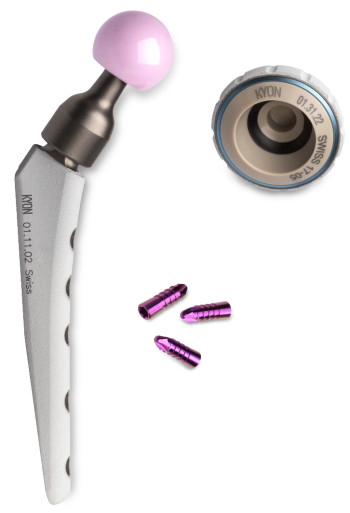
The latest generation of cementless hip prostheses for permanent use on dogs and cats from the Swiss company KYON. At the heart of the innovative technology is a friction partner made of VESTAKEEP PEEK biomaterial from Evonik. Image courtesy of KYON.
The hip dysplasia treatment method of the Swiss company KYON is based on a novel cementless hip prosthesis. It was designed from a combination of several high-performance materials, each of which is capable of performing all its functions in the best possible way.
"One of the weak points of hip prostheses has always been the tribology between the head and the cup. This is the crucial point where damage due to frictional losses accumulates in the form of wear particles with the millions of annual movement cycles of an active dog. The performance of the tribological pair, therefore, significantly determines the lifespan of a hip prosthesis," explained Dr. Slobodan Tepic, founder of KYON. "In developing our system, we were always driven by the desire to permanently reduce the suffering of the animals. For us, this meant devising a hip prosthesis system that does not require any subsequent revision."
The result of over two decades of research and development by KYON is the latest generation of cementless hip prostheses for permanent use. At the heart of the innovative technology is a friction partner—a so-called inlay made of Evonik's VESTAKEEP PEEK biomaterial—with an additional carbon fiber-reinforced PEEK ring between the ceramic head and the cup.
"VESTAKEEP PEEK is a proven high-performance polymer for implants in human medicine offering outstanding tribological properties. Unpublished data suggests that linear wear is reduced by a factor of seven with ceramic on PEEK when compared to conventional pairing, which is why we have based our latest generation of hip prostheses on this material combination," explained Guy C. Spörri, CEO of KYON.
Approximately 6,000 successful hip replacement procedures without a single revision due to inlay wear and as many satisfied dogs—including true champions (agility dogs)—and five years of detailed documentation confirm the success of KYON's hip prosthesis system relying on PEEK. The polymer experts from Evonik want to build on this success and are aiming to make it possible to use PEEK in human joint prostheses.
Knowledge Transfer from Veterinary to Human Medicine
Joints are complex movement systems that fulfill important anatomical functions. At the Medical Device Competence Center in Birmingham, Alabama, Evonik is pursuing the approach of analyzing the weak points of the joint prosthesis systems already established on the market in human medicine and developing a solution with its high-performance PEEK polymer. Supported by the knowledge gained from our collaboration with KYON and our many years of materials expertise in polymer design, we are examining the use of VESTAKEEP in human joint prostheses to bring about a significant improvement for the patient. For example, we have learned to understand PEEK as a material component in complex joint prosthesis systems that can be integrated into existing technologies according to the modular principle.
Hip joint replacement operations have long been among the most standard operations. In the group of OECD (Organisation for Economic Co-operation and Development) countries, an average of 182 procedures were performed per 100,000 population in 2017, according to the Health at a Glance 2017 Report. This figure was 30 percent higher than ten years earlier. The current technologies are convincing in terms of availability and reliability, as well as professional and experienced handling by orthopedic specialists.
Leveraging Evonik’s PEEK material, we believe we can extend the service life of existing hip systems and thus improve the quality of life for patients.
PEEK for Joint Prostheses

At the Medical Device Competence Center in Birmingham, Alabama, Evonik is pursuing the approach of analyzing the weak points of the joint prosthesis systems already established on the market in human medicine and developing a solution with the high-performance PEEK polymer. Image courtesy of Evonik.
In terms of materials or functional requirements, the human hip joint prosthesis barely differs from those used in veterinary orthopedics. Similarly, the friction partner between the head and the cup anchored in the bone is a primary weak point of today's technologies. Evonik’s tribological PEEK biomaterial could make the decisive difference in the future and extend the life of a hip prosthesis fourfold. If it were, millions of patients worldwide could do without years of pain-relieving therapies. These are often necessary to reach a certain age for surgery, so that the probability of a risky revision at an advanced age can be reduced.
The promising patient perspective and the success story of KYON give Evonik's polymer experts the necessary drive to push the PEEK biomaterial for use in human joint prostheses through all regulatory measures with potential partners. The specialty chemicals company's determination is expressed in its close cooperation with the Massachusetts General Hospital (MGH) in Boston. "We draw on the expertise of medical specialists from the MGH's globally recognized center for knee and hip replacement, test our material for tribological properties in their professional laboratories, and receive valuable feedback that always takes us one step further," explained Kenneth Ross, head of Evonik's medical technology business in America.
The use of VESTAKEEP PEEK as a material component in human joint prostheses is an innovative scenario and once again demonstrates the possibilities of high-performance materials in modern medicine. If the breakthrough is successful, a new quality in the treatment of hip arthrosis would be available to human medicine. Considering the more than 300 million cases of hip and knee osteoarthritis worldwide in 2017, as published by the Annals of the Rheumatic Diseases magazine, this would be a significant medical achievement.
Marc Knebel is global segment head medical systems—head of VESTAKEEP Europe bei Evonik Operations GmbH. He started his career in 1998 at Quadrant Engineering Plastics AG (Mitsubishi Chemical Advanced Materials) within different roles, including global market segment manager for medical and life science applications. Since 2008, Knebel has worked for the Evonik Division Smart Materials in the Business Line High Performance Polymers. He developed and launched the product portfolio VESTAKEEP for implant and dental applications and is currently heading the Global Medical Systems Team. He graduated from Darmstadt University of Applied Sciences with a plastics engineering degree and from Ludwigshafen University of Business and Society with a degree in Economics.
Additional Insights

Marc Knebel, head of the Medical Systems Market Segment at Evonik. Image courtesy of Evonik.
In the following brief Q&A, Guy C. Spörri, CEO of KYON and Marc Knebel, address a number of questions about the project.
Q: What are the hurdles of such a technology transfer in terms of size or weight load?
Guy C. Spörri: The weight of a dog is between 3 and 70 kg, depending on the breed, and is significantly lower compared to humans with their average of 80 kg. But if you look at the movement cycles, our prostheses are subjected to much more stress than those used in human medicine. The number of movement cycles in dogs and cats is ten times higher than in humans. We offer our hip prostheses in different sizes, since size is a relevant requirement for pets. From the KYON perspective, we can therefore build on the in-depth knowledge in size scaling of our technology. Currently, we are even developing a small hip prosthesis model, which is especially interesting for markets such as Japan or big cities, where small or toy breed dogs are common.
Marc Knebel: One of the success factors of VESTAKEEP is its outstanding tribological behavior, in addition to the biocompatibility required in medical technology. It is the decisive feature for compensating frictional stress between the hip system components during millions of movement cycles. PEEK-based implants can therefore withstand even extreme physical stress, such as during sports, very well.

Guy C. Spörri, CEO at KYON. Image courtesy of KYON.
Q: How do you assess the chances of technology transfer from veterinary to human medicine?
Spörri: The hip prosthesis system developed and marketed by KYON is a sixth-generation technology. More than 20 years of experience and external expertise have resulted in the innovation. Our cementless system based on PEEK is probably the best on the market today, even in direct comparison to human hip prostheses.
Knebel: The regulatory approval procedure in human medicine is strictly oriented toward added value for patients. We have to provide sufficient evidence of this added value (i.e., the extension of the service life of a hip prosthesis and thus a significant reduction in revision procedures) in order to win partners for the further development steps. For this reason, we are cooperating with the Center for Knee and Hip Replacement at Massachusetts General Hospital (MGH) in Boston.
Whether internal fixation of bone fractures, treatment of cruciate rupture, or total hip replacement, many of today's veterinary treatment methods would not be conceivable at all without the underlying highly regulated human medicine. As a rule, veterinary medicine participates in the achievements of medical devices approved for humans by the FDA (U.S. Food and Drug Administration) or the PMDA (Japan’s Pharmaceuticals and Medical Devices Agency). The polymer experts from the specialty chemicals company Evonik wanted to see it happen the other way around. According to their idea, high-performance, proven materials used in the treatment of hip dysplasia in dogs and cats should be used in human medicine. The success of the innovative technology so far could prove them right.
Innovative Hip Prostheses for Pets

The latest generation of cementless hip prostheses for permanent use on dogs and cats from the Swiss company KYON. At the heart of the innovative technology is a friction partner made of VESTAKEEP PEEK biomaterial from Evonik. Image courtesy of KYON.
"One of the weak points of hip prostheses has always been the tribology between the head and the cup. This is the crucial point where damage due to frictional losses accumulates in the form of wear particles with the millions of annual movement cycles of an active dog. The performance of the tribological pair, therefore, significantly determines the lifespan of a hip prosthesis," explained Dr. Slobodan Tepic, founder of KYON. "In developing our system, we were always driven by the desire to permanently reduce the suffering of the animals. For us, this meant devising a hip prosthesis system that does not require any subsequent revision."
The result of over two decades of research and development by KYON is the latest generation of cementless hip prostheses for permanent use. At the heart of the innovative technology is a friction partner—a so-called inlay made of Evonik's VESTAKEEP PEEK biomaterial—with an additional carbon fiber-reinforced PEEK ring between the ceramic head and the cup.
"VESTAKEEP PEEK is a proven high-performance polymer for implants in human medicine offering outstanding tribological properties. Unpublished data suggests that linear wear is reduced by a factor of seven with ceramic on PEEK when compared to conventional pairing, which is why we have based our latest generation of hip prostheses on this material combination," explained Guy C. Spörri, CEO of KYON.
Approximately 6,000 successful hip replacement procedures without a single revision due to inlay wear and as many satisfied dogs—including true champions (agility dogs)—and five years of detailed documentation confirm the success of KYON's hip prosthesis system relying on PEEK. The polymer experts from Evonik want to build on this success and are aiming to make it possible to use PEEK in human joint prostheses.
Knowledge Transfer from Veterinary to Human Medicine
Joints are complex movement systems that fulfill important anatomical functions. At the Medical Device Competence Center in Birmingham, Alabama, Evonik is pursuing the approach of analyzing the weak points of the joint prosthesis systems already established on the market in human medicine and developing a solution with its high-performance PEEK polymer. Supported by the knowledge gained from our collaboration with KYON and our many years of materials expertise in polymer design, we are examining the use of VESTAKEEP in human joint prostheses to bring about a significant improvement for the patient. For example, we have learned to understand PEEK as a material component in complex joint prosthesis systems that can be integrated into existing technologies according to the modular principle.
Hip joint replacement operations have long been among the most standard operations. In the group of OECD (Organisation for Economic Co-operation and Development) countries, an average of 182 procedures were performed per 100,000 population in 2017, according to the Health at a Glance 2017 Report. This figure was 30 percent higher than ten years earlier. The current technologies are convincing in terms of availability and reliability, as well as professional and experienced handling by orthopedic specialists.
Leveraging Evonik’s PEEK material, we believe we can extend the service life of existing hip systems and thus improve the quality of life for patients.
PEEK for Joint Prostheses

At the Medical Device Competence Center in Birmingham, Alabama, Evonik is pursuing the approach of analyzing the weak points of the joint prosthesis systems already established on the market in human medicine and developing a solution with the high-performance PEEK polymer. Image courtesy of Evonik.
The promising patient perspective and the success story of KYON give Evonik's polymer experts the necessary drive to push the PEEK biomaterial for use in human joint prostheses through all regulatory measures with potential partners. The specialty chemicals company's determination is expressed in its close cooperation with the Massachusetts General Hospital (MGH) in Boston. "We draw on the expertise of medical specialists from the MGH's globally recognized center for knee and hip replacement, test our material for tribological properties in their professional laboratories, and receive valuable feedback that always takes us one step further," explained Kenneth Ross, head of Evonik's medical technology business in America.
The use of VESTAKEEP PEEK as a material component in human joint prostheses is an innovative scenario and once again demonstrates the possibilities of high-performance materials in modern medicine. If the breakthrough is successful, a new quality in the treatment of hip arthrosis would be available to human medicine. Considering the more than 300 million cases of hip and knee osteoarthritis worldwide in 2017, as published by the Annals of the Rheumatic Diseases magazine, this would be a significant medical achievement.
Marc Knebel is global segment head medical systems—head of VESTAKEEP Europe bei Evonik Operations GmbH. He started his career in 1998 at Quadrant Engineering Plastics AG (Mitsubishi Chemical Advanced Materials) within different roles, including global market segment manager for medical and life science applications. Since 2008, Knebel has worked for the Evonik Division Smart Materials in the Business Line High Performance Polymers. He developed and launched the product portfolio VESTAKEEP for implant and dental applications and is currently heading the Global Medical Systems Team. He graduated from Darmstadt University of Applied Sciences with a plastics engineering degree and from Ludwigshafen University of Business and Society with a degree in Economics.
Additional Insights

Marc Knebel, head of the Medical Systems Market Segment at Evonik. Image courtesy of Evonik.
Q: What are the hurdles of such a technology transfer in terms of size or weight load?
Guy C. Spörri: The weight of a dog is between 3 and 70 kg, depending on the breed, and is significantly lower compared to humans with their average of 80 kg. But if you look at the movement cycles, our prostheses are subjected to much more stress than those used in human medicine. The number of movement cycles in dogs and cats is ten times higher than in humans. We offer our hip prostheses in different sizes, since size is a relevant requirement for pets. From the KYON perspective, we can therefore build on the in-depth knowledge in size scaling of our technology. Currently, we are even developing a small hip prosthesis model, which is especially interesting for markets such as Japan or big cities, where small or toy breed dogs are common.
Marc Knebel: One of the success factors of VESTAKEEP is its outstanding tribological behavior, in addition to the biocompatibility required in medical technology. It is the decisive feature for compensating frictional stress between the hip system components during millions of movement cycles. PEEK-based implants can therefore withstand even extreme physical stress, such as during sports, very well.

Guy C. Spörri, CEO at KYON. Image courtesy of KYON.
Spörri: The hip prosthesis system developed and marketed by KYON is a sixth-generation technology. More than 20 years of experience and external expertise have resulted in the innovation. Our cementless system based on PEEK is probably the best on the market today, even in direct comparison to human hip prostheses.
Knebel: The regulatory approval procedure in human medicine is strictly oriented toward added value for patients. We have to provide sufficient evidence of this added value (i.e., the extension of the service life of a hip prosthesis and thus a significant reduction in revision procedures) in order to win partners for the further development steps. For this reason, we are cooperating with the Center for Knee and Hip Replacement at Massachusetts General Hospital (MGH) in Boston.
























