Michael Barbella, Managing Editor02.18.15
"The world hates change, yet it is the only thing that has brought progress.” — Charles Kettering
Some homecoming.
Steve Jobs was back on top at Apple Inc., having returned from a 12-year exile in the summer of 1997 to save the company he co-founded. The reunion, though, was hardly joyous: In his absence, the once quixotic firm had gone woefully astray, hemorrhaging profits, bosses, market share, and most regrettably, influence as it struggled to overcome the loss of its graphic user interface monopoly in 1994.
Jobs’s successors largely were responsible for the company’s downward spiral—none could match his incredible vision or innate ability to truly comprehend the relationships between wildly disparate life experiences and technological advancements. Their best attempts resulted in a spate of failed products—i.e., the Newton, the Pippin, the Twentieth Anniversary Macintosh—that eventually cost Apple billions of dollars in lost sales.
Jobs had known his company was in trouble but the damage was far worse than he realized. Stock was trading at historic lows ($3.19), and Q1 1997 sales were off by a staggering $708 million. Under CEO Gil Amelio’s 500-day watch, Apple lost more than $1 billion in revenue.
Jobs wasted no time strategizing the company’s resurrection. Upon his return, he hired renowned advertising firm TBWA\Chiat\Day, the creative genius behind Nissan’s famous “Toys” spot, to breathe new life into the Apple brand. Within weeks, the Los Angeles, Calif., agency had conceived the iconic “Think Different” campaign that ultimately launched Apple’s impressive comeback.
The 60-second television commercial TBWA\Chiat\Day created was simple, yet powerful, consisting solely of black-and-white video footage of noteworthy historical figures—trailblazers like Albert Einstein, the Rev. Martin Luther King Jr., Thomas Edison, Ted Turner, Mahatma Gandhi, Amelia Earhart, Martha Graham and Pablo Picasso. As the video plays, actor Richard Dreyfuss explains the common thread linking each of their lives: “Here’s to the crazy ones. The misfits. The rebels. The troublemakers. The round pegs in the square holes. The ones who see things differently. They’re not fond of rules and they have no respect for the status quo. You can quote them, disagree with them, glorify or vilify them. About the only thing you can’t do is ignore them because they change things. They push the human race forward. While some may see them as the crazy ones, we see genius. Because the people who are crazy enough to think they can change the world are the ones that do.”
The commercial ends with the image of a young girl, Shaan Sahota, opening her closed eyes as she realizes, perhaps for the very first time, all the possibilities within her reach. The screen then fades to black, where the words “Think different” appear below the Apple logo.
Think different.
Sage advice, surely, considering the impact it has had on society. Without “crazy ones” like Einstein, King, Picasso, Edison, Gandhi and yes, Jobs (he was, no doubt, insane enough to set up an online music store), life on Planet Earth would be quite different. No electricity, cameras or theory of relativity, no segregation or female pilots, no iTunes, iPod, iPad or iPhone.
While it’s an admirable concept, thinking differently is a tall order for most large companies because it requires they betray their own DNA. Few, if any, organizations are willing to gamble with the genetic jackpot of accountability, reliability and environmental inertia. Thinking differently necessitates change, and enterprises rarely change their fundamental structural composition—they are, after all, bound by the limits of human nature.
“Once the human mind has set out to do something, or has gotten in the habit of doing something,” serial entrepreneur Jeff Stible told The Atlantic, “changing it is very hard.”
So hard that even multinationals like Coca-Cola are bound to fail. The beverage behemoth tried changing the recipe of its classic soft drink in the mid-1980s but encountered such a public backlash that it ditched its revamped product and reissued its original formula.
Change isn’t much easier to come by in the medtech industry, where familiarity and tradition generally prevail over non-conformity and outside-the-box thinking. Case in point: Drug delivery companies like Pfizer, Eli Lilly and Company, Novartis and others have been using DC 360—a non-human-grade silicone oil—on the insides of syringes for nearly half a century to ensure the smooth movement of the plunger during injection. The oil, however, can clump certain bioengineered proteins, it can turn certain drugs cloudy and it also can alter the shelf life of some pharmaceuticals.
Yet companies continue to use the oil.
“About two years ago, I chaired a discussion group where people talked about all the problems with a certain technology,”recalled David Opie, Ph.D. “The morning of that conference, we heard people talk about alternatives to that technology, but no one was using those alternatives. I asked the panel why they were still discussing the problems with the original technology when we heard about alternatives that don‘t have the same issues. One guy said, ‘No one’s ever been fired for choosing the status quo.’ That’s it in a nutshell. No one is willing to put their career on the line to risk a product line in which millions of dollars are invested for the hope of something better because they have the devil they know. They have problems with the current technology and they don’t know what problems might arise when they try to implement the new technology. People see that unknown as a risk to their career and a risk to the product line. It’s that risk that really steers the decision-making and adoption of new technologies across the board. Change is hard ... no one wants to be first.”
Certainly not. But medtech companies may have no other choice—cost pressures, federal/international regulations, environmental concerns and device complexity are nudging them toward previously uncharted territory in sterilization techniques.
Blazing new trails are technologies such as nitrogen dioxide (NO2), X-ray and supercritical carbon dioxide (SCCO2), the latter of which is used mostly in the orthopedic industry to sterilize bone, tendon, skin and other allograft tissue. Developed by Lansing, N.Y.-based NovaSterilis Inc., SCCO2 is neither a gas nor liquid, but rather carbon dioxide in a “fluid state,” the point at which its temperature and pressure are equal to or greater than the critical point (31.1 degrees Celsius, 74 atm).
By falling within the gas-liquid spectrum mid-point, supercritical fluids inherit the talents of both forms of matter—they can diffuse through solids like a gas, and dissolve materials like a liquid. Such non-committal, along with its density, solvency, gas-like viscosity, compressibility and low surface tension, enables SCCO2 to better penetrate bone and allograft tissue without damaging their structural or chemical integrity, the company claims. As a result, the process completely kills infectious organisms that might be missed by traditional bone sterilization methods like gamma radiation and cold washings with disinfecting solutions.
Since it converts to its supercritical (fluid) stage at a relatively low pressure and temperature, SCCO2 is ideal for materials that are heat-sensitive or reactive with other sterilization methods. It is non-toxic, non-flammable, chemically inert, comparably inexpensive, and can easily be removed from a system by simple depressurization.
NovaSterilis’s Nova 2200 features a 20-liter sterilization chamber in which up to 60 individual allografts (transplant tissues) can be purified. To deactivate spores as well as live bacteria, the company provides sheets of paper treated with a mix of chemical additives including an acid that can be dissolved into the supercritical SCCO2 sterilizing fluid, much like detergent in a washing machine. The fluid penetrates bacterial cells and kills them.
SCCO2 sterilization slowly is gaining popularity and acceptance in the medtech industry, having been used to sanitize regenerative materials, biologic scaffolds and resorbable polymers ranging from poly-L-lactide and poly-D-lactide to L-lactide, DL-lactide and L-lactide/glycolide. Sutures could soon be added to the mix—last spring, NovaSterilis received a patent to remove toxic ethylene oxide (EO) residuals from absorbable sutures following sterilization. In working to obtain the patent, the company eliminated the need for EO sterilization altogether, achieving a Sterility Assurance Level of 10-6, with SCCO2 maintaining the sutures’ integrity.
“This new application can be applied across many classes of products that are sterilized with EO, potentially improving the performance of the product and outcomes of procedures utilizing these devices,” NovaSterilis President David Burns said upon the issuance of U.S. Patent No. 8658091. “We are confident this process can be expanded to a broad range of products and chemicals including, for example, the removal of residual solvents from pharmaceuticals.”
A legion of possibilities truly exists as the company attempts to branch into other sectors: Its SCCO2 technology already has infiltrated supranamics manufacturing, industrial degreasing, silicon wafer cleaning and textile dyeing. Vaccine/pharmaceutical purification and materials coatings are on the horizon as well.
The technology also is becoming more widespread within healthcare. More than a half-dozen U.S. tissue banks use SCCO2 to clean bone, tendon and other products; Australian Biotechnologies Pty. Ltd. received government approval in 2012 to market SCCO2-sterilized cortical and cancellous bone products; and a Dutch tissue processor/contract manufacturer is distributing SCCO2-treated allografts in Europe, India, China and Malaysia.
Indeed, the supercritical sterilization approach is gaining quite a toehold in the medtech arena. Yet many tissue banks are hesitant to invest in the new technology even though it can help suppliers significantly reduce costs by bringing the sterilization process in-house. In addition, the SCCO2 process—like gamma irradiation—can be performed while samples are sealed within their final packaging, reducing the opportunity for the reintroduction of contaminants.
Nevertheless, surgeons are optimistic about the future of supercritical sterilization. “I see the process becoming a standard in the industry,” one medtech executive speculated.
X-rays carry the same potential.
X-radiation is a viable alternative to hydrogen peroxide, steam and other traditional cleaning methods due to advancements in high-energy, high-power electron accelerators. Belgian firm Ion Beam Applications S.A. (IBA) is a pioneer in the field, having developed a particle accelerator in 1992 from a patented concept of the French Atomic Energy Commission. The company offers customers an X-ray sterilization solution called eXelis based on its high-power, high-energy Rhodotron accelerator, which provides one or several beam outputs from 2 to 10 MeV, with power ranging from 35 to 700 kW. IBA says eXelis helps improve the cost competitiveness of X-ray sterilization by reducing dose uniformity ratio (DUR) levels (the ratio between the maximum and minimum doses required to effectively process a product). Data supplied by IBA claim X-radiation can achieve a DUR of 1.25 for pallet loads of low-density materials compared with a DUR of 1.45 for the same low-density loads in a cobalt-60 gamma radiation facility.
X-ray sterilization offers several advantages over traditional methods like gamma radiation and ethylene oxide (EO). For starters, X-ray photons are more concentrated and directed than the widely scattered cobalt-60 gamma rays. X-ray systems also penetrate full pallets of product better than gamma and electron beam methods, hence their lower DURs. Pallets in X-ray facilities typically are irradiated from one side as they pass in front of a long, vertically-oriented target and irradiated on the opposite side to obtain a nearly uniform dose in the vertical direction.
The concentration of X-ray photons significantly reduces product exposure times, thereby minimizing or altogether eliminating damage to certain medical device materials. X-ray processing, in fact, can be used to chemically bond (cross-link) polymer chains together, effectually making plastics more resistant to stress and extreme conditions.
Despite its litany of benefits, however, X-ray sterilization is decidedly under-used due largely to a lack of capacity. The industry’s only large-scale medical device X-ray sterilization facility is owned by Synergy Health plc and located in Däniken, Switzerland. The British outsourcing provider of sterilization, laboratory, linen management, hospital and reusable surgical solution services inherited the plant with its 2012 purchase of Leoni Studer Hard AG for 47.6 million euros ($63.4 million). Featuring a 700kW Rhodotron electron beam accelerator, the facility can process up to 80,000 pallets annually.
“X-ray is an old technology, it’s been out for decades, but using it for sterilization purposes is a fairly new application. It’s the new kid on the block,” noted Mark Berger, Synergy’s business development manager. “Gamma radiation and EO processing have been around for 50 years and have built up such a reputation that they are the default methods. But there are other options out there.
Materials and coatings companies are partnering with sterilization providers and running tests to see what materials work best with the different [sterilization] processes. We have materials companies in the U.S. contacting our X-ray group in Switzerland to ask whether they can send the group materials so they can put together a white paper on the X-ray process and market the material. Companies are so used to using gamma or EO that they automatically think one of them will work for sterilization. Ninety-nine percent of the time they do work, but it’s not always the most cost-effective way to sterilize a product.”
Or the most suitable for the material.
Porous polyactone scaffolds, for example, best tolerate gas plasma processing while calcium phosphate bioceramics prefer EO (autoclaving significantly reduces its compressive strength). Gamma radiation is ideal for cross-linking ultra high molecular weight polyethylene (UHWPE) to reduce wear in acetabular hip liners, but the process can change the molecular weight of polylactic acid biodegradables (anchors for tendons and bone screws). Many newer orthopedic materials, in fact—drug/device combinations, hydrogels, polysaccharides and poly(amino acids)—are predisposed to room temperature sterilization processes.
Noxilizer Inc. provides such an alternative, disinfecting products at room temperature with minimal vacuum using nitrogen dioxide technology. The company’s NO2 sterilization process typically lasts less than three hours (including the aeration phase); vacuum can be used to provide a faster cycle for devices that can withstand pressure changes, but the NO2 gas readily diffuses the packaging when minimal vacuum is used to exchange process gases in the chamber.
One of the main advantages of NO2 technology is its compatibility with temperature-sensitive materials and drug-device combination products. Another major benefit of NO2 sterilization is its short aeration and cycle times. A routine cycle lasts 60-90 minutes (including 15 minutes for aeration), thus enabling a small sterilizer (about 360 liters of usable volume) to disinfect an entire pallet of product in an eight-hour shift and return it immediately to inventory. Such truncated cycle times are particularly important to orthopedic device manufacturers that customize implants for patients, as they generally are strapped for time to 3-D print, package and return products, industry experts claim.
Perhaps the greatest advantage of NO2 technology, however, is the potential for in-house processing, which can eliminate the transportation and inventory carrying costs typically incurred by higher volume manufacturers. NO2 gas is non-carcinogenic and non-flammable, making it considerably safer to set up in-house than gamma radiation, which requires a shielded storage room (wet or dry), a radiation shield (a thick concrete wall) surrounding the irradiation room, a control console room, and a product transport system. In addition, Noxilizer’s RTS 360 Industrial NO2 Sterilizer is designed to support lean manufacturing processes, and may be placed in line with assembly and packaging operations.
“Cost pressures within the orthopedics market are growing. This trend must be considered in orthopedic product development and manufacturing, and this is where sterilization can play a role,” said Opie, senior vice president of research and development at Noxilizer, a Baltimore, Md.-based seller of sterilization equipment and a contract NO2 sterilization provider to the medical device, biotechnology and pharmaceutical industries. “NO2 can help orthopedic manufacturers reduce turnaround of products and carrying costs by allowing small batches to be processed in-house. Costs per pallet are lower in-house than with a contract sterilizer. Technologies like gamma and EO are not easily brought in house—gamma requires building a radiation shield and EO has safety concerns. Outcomes-based reimbursement and other changes also will place cost pressures on hospitals performing surgical procedures. These cost pressures will impact the medical device supply chain, which includes the sterilization process. But these cost pressures and advancements like biodegradable implants and 3-D printing are giving NO2 an opportunity to grow. And that’ s good because there are lots of opportunities to grow—either from early adoption, companies looking to save money or those who truly want to change.”
Many customers, though, remain loyal to old favorites like gamma and EO. Sterigenics International LLC, for instance, spent more than $800 million last year beefing up its gamma capabilities, purchasing Canadian medical isotope/gamma technology provider Nordion Inc.; Mulberry, Fla.-based Food Technology Service Inc.; and Italian firm Gammarad. It also invested more than $10 million to expand its EO processing capacity in its Smyrna, Ga., Grand Prairie, Texas, and Petit-Rechain, Belgium, facilities.
For Sterigenics, the money is well spent. Despite their drawbacks, gamma radiation and ethylene oxide are still the preferred choice of medical device manufacturers, with companies nearly evenly split between both methods. Their appeal is multifaceted, though each has specific advantages over the other.
EO, for instance, is compatible with a wide range of materials and penetrates its target better than dry heat or steam. It also has the least impact on final material properties, which is particularly important for color-coded products.
“Manufacturers need to consider how the sterilization method will affect their product, such as color adonization or change to product composition,” explained Victoria Hughes, vice president of quality systems at Millstone Medical Outsourcing, a Fall River, Mass.-based medical device packaging provider. “Those colors usually indicate size. Companies need to consider product impact when weighing sterilization options.”
Ethylene oxide also is an effectual killer, eliminating all known viruses, bacteria, fungi and bacterial spores (typically the most sterilization-resistant type of microbe).
Gamma radiation is an efficient germ-slayer as well —the process disrupts pathogens’ DNA bond, preventing the cellular division necessary to sustain life. But the method leaves no harmful residue and is more permeable than EO, enabling products to be sterilized while sealed inside their final packaging (thus allowing device manufacturers to skip the post-sterilization drying and packaging step). And, since the radiation reaches the bulk inner material of the device, there is little, if any, risk of contamination within the body as the product degrades.
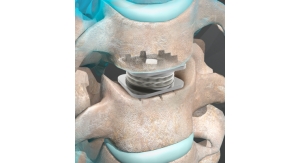
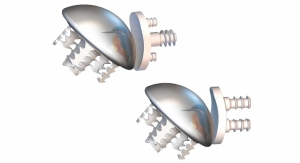
Gamma’s penetrability is specially appealing to artificial joint makers, who constantly are striving to build longer-lasting products. The process has no lasting impact on metals like titanium, stainless steel and chromium but it can change the molecular structure of polymers, leading to a better implant material for acetabular hip cups.
Radiation—either gamma or e-beam—is used in crosslinking, a process where long chains of polymers are linked together to increase its mass. Crosslinking also can make a polymer more scratch resistant and chemically immune, more responsive to higher temperatures, and improve shape memory retention (elastomers may be crosslinked to a slight degree to give them “memory”; they usually will return to their original shape after expansion).
“Crosslinking is well-known in the plastics industry to strengthen different types of polymers. The orthopedic industry is really looking to reduce the wear of the material,” said Scott Goetz, sales manager at Deerfield, Ill.-based Sterigenics, a contract sterilization provider to the medical device, pharmaceutical, food safety and high performance/specialty materials industries. “If you take the plastic or metal material and you rotate it on itself, eventually you’re going to wear it down and pieces of the plastic is going to go into the body and the body will reject it. The crosslinking actually takes the bonds of the ultra high molecular weight polymer and tightens it so you don’t have that wearing, or if you do, it takes a long longer to wear. The majority of ultra high molecular weight materials is crosslinked in gamma irradiation—it’s a more straightforward process. Currently, most of the implantable ultra high molecular weight polyethylene is radiation crosslinked, which reduces wear by 90 percent and will last the patient a lifetime. The latest trend in orthopedic implants is the introduction of Vitamin E-impregnated ultra high molecular weight polyethylene. The Vitamin E acts as an anti-oxidant in the polyethylene and therefore has less chance of rejection by the body, but at the same time it changes the process of radiation crosslinking that needs to be performed.”
* * *
Old habits die hard. Familiarity and tradition breed comfort, and comfort—in the board room, anyway—can beget institutional inertia. History is littered with the corpses of organizations that became a bit too comfortable with custom and resisted change (Radio Shack and Kodak are prime examples).
The medtech industry has never been too enamored of change. But companies increasingly are recognizing the benefits of innovative thinking as they attempt to keep pace with market changes. Nevertheless, there always will be some allegiance to routine and custom—even Jobs himself couldn’t escape the routine of daily product/management meetings and design lab time.
Sterilizers are striking a balance between the past and future, remaining loyal to gamma and EO methods for regulatory and manufacturing efficiency purposes, but exploring safer, less expensive alternatives. As Millstone’s Hughes noted: “In the knee, hip and shoulder areas, companies are not looking to get away from gamma or EO. They’ve been historically effective processes. But for newer devices that have electronics or are combination-type products, that’s where companies are looking into the newer methods.”
Some homecoming.
Steve Jobs was back on top at Apple Inc., having returned from a 12-year exile in the summer of 1997 to save the company he co-founded. The reunion, though, was hardly joyous: In his absence, the once quixotic firm had gone woefully astray, hemorrhaging profits, bosses, market share, and most regrettably, influence as it struggled to overcome the loss of its graphic user interface monopoly in 1994.
Jobs’s successors largely were responsible for the company’s downward spiral—none could match his incredible vision or innate ability to truly comprehend the relationships between wildly disparate life experiences and technological advancements. Their best attempts resulted in a spate of failed products—i.e., the Newton, the Pippin, the Twentieth Anniversary Macintosh—that eventually cost Apple billions of dollars in lost sales.
Jobs had known his company was in trouble but the damage was far worse than he realized. Stock was trading at historic lows ($3.19), and Q1 1997 sales were off by a staggering $708 million. Under CEO Gil Amelio’s 500-day watch, Apple lost more than $1 billion in revenue.
Jobs wasted no time strategizing the company’s resurrection. Upon his return, he hired renowned advertising firm TBWA\Chiat\Day, the creative genius behind Nissan’s famous “Toys” spot, to breathe new life into the Apple brand. Within weeks, the Los Angeles, Calif., agency had conceived the iconic “Think Different” campaign that ultimately launched Apple’s impressive comeback.
The 60-second television commercial TBWA\Chiat\Day created was simple, yet powerful, consisting solely of black-and-white video footage of noteworthy historical figures—trailblazers like Albert Einstein, the Rev. Martin Luther King Jr., Thomas Edison, Ted Turner, Mahatma Gandhi, Amelia Earhart, Martha Graham and Pablo Picasso. As the video plays, actor Richard Dreyfuss explains the common thread linking each of their lives: “Here’s to the crazy ones. The misfits. The rebels. The troublemakers. The round pegs in the square holes. The ones who see things differently. They’re not fond of rules and they have no respect for the status quo. You can quote them, disagree with them, glorify or vilify them. About the only thing you can’t do is ignore them because they change things. They push the human race forward. While some may see them as the crazy ones, we see genius. Because the people who are crazy enough to think they can change the world are the ones that do.”
The commercial ends with the image of a young girl, Shaan Sahota, opening her closed eyes as she realizes, perhaps for the very first time, all the possibilities within her reach. The screen then fades to black, where the words “Think different” appear below the Apple logo.
Think different.
Sage advice, surely, considering the impact it has had on society. Without “crazy ones” like Einstein, King, Picasso, Edison, Gandhi and yes, Jobs (he was, no doubt, insane enough to set up an online music store), life on Planet Earth would be quite different. No electricity, cameras or theory of relativity, no segregation or female pilots, no iTunes, iPod, iPad or iPhone.
While it’s an admirable concept, thinking differently is a tall order for most large companies because it requires they betray their own DNA. Few, if any, organizations are willing to gamble with the genetic jackpot of accountability, reliability and environmental inertia. Thinking differently necessitates change, and enterprises rarely change their fundamental structural composition—they are, after all, bound by the limits of human nature.
“Once the human mind has set out to do something, or has gotten in the habit of doing something,” serial entrepreneur Jeff Stible told The Atlantic, “changing it is very hard.”
So hard that even multinationals like Coca-Cola are bound to fail. The beverage behemoth tried changing the recipe of its classic soft drink in the mid-1980s but encountered such a public backlash that it ditched its revamped product and reissued its original formula.
Change isn’t much easier to come by in the medtech industry, where familiarity and tradition generally prevail over non-conformity and outside-the-box thinking. Case in point: Drug delivery companies like Pfizer, Eli Lilly and Company, Novartis and others have been using DC 360—a non-human-grade silicone oil—on the insides of syringes for nearly half a century to ensure the smooth movement of the plunger during injection. The oil, however, can clump certain bioengineered proteins, it can turn certain drugs cloudy and it also can alter the shelf life of some pharmaceuticals.
Yet companies continue to use the oil.
“About two years ago, I chaired a discussion group where people talked about all the problems with a certain technology,”recalled David Opie, Ph.D. “The morning of that conference, we heard people talk about alternatives to that technology, but no one was using those alternatives. I asked the panel why they were still discussing the problems with the original technology when we heard about alternatives that don‘t have the same issues. One guy said, ‘No one’s ever been fired for choosing the status quo.’ That’s it in a nutshell. No one is willing to put their career on the line to risk a product line in which millions of dollars are invested for the hope of something better because they have the devil they know. They have problems with the current technology and they don’t know what problems might arise when they try to implement the new technology. People see that unknown as a risk to their career and a risk to the product line. It’s that risk that really steers the decision-making and adoption of new technologies across the board. Change is hard ... no one wants to be first.”
Certainly not. But medtech companies may have no other choice—cost pressures, federal/international regulations, environmental concerns and device complexity are nudging them toward previously uncharted territory in sterilization techniques.
Blazing new trails are technologies such as nitrogen dioxide (NO2), X-ray and supercritical carbon dioxide (SCCO2), the latter of which is used mostly in the orthopedic industry to sterilize bone, tendon, skin and other allograft tissue. Developed by Lansing, N.Y.-based NovaSterilis Inc., SCCO2 is neither a gas nor liquid, but rather carbon dioxide in a “fluid state,” the point at which its temperature and pressure are equal to or greater than the critical point (31.1 degrees Celsius, 74 atm).
By falling within the gas-liquid spectrum mid-point, supercritical fluids inherit the talents of both forms of matter—they can diffuse through solids like a gas, and dissolve materials like a liquid. Such non-committal, along with its density, solvency, gas-like viscosity, compressibility and low surface tension, enables SCCO2 to better penetrate bone and allograft tissue without damaging their structural or chemical integrity, the company claims. As a result, the process completely kills infectious organisms that might be missed by traditional bone sterilization methods like gamma radiation and cold washings with disinfecting solutions.
Since it converts to its supercritical (fluid) stage at a relatively low pressure and temperature, SCCO2 is ideal for materials that are heat-sensitive or reactive with other sterilization methods. It is non-toxic, non-flammable, chemically inert, comparably inexpensive, and can easily be removed from a system by simple depressurization.
NovaSterilis’s Nova 2200 features a 20-liter sterilization chamber in which up to 60 individual allografts (transplant tissues) can be purified. To deactivate spores as well as live bacteria, the company provides sheets of paper treated with a mix of chemical additives including an acid that can be dissolved into the supercritical SCCO2 sterilizing fluid, much like detergent in a washing machine. The fluid penetrates bacterial cells and kills them.
SCCO2 sterilization slowly is gaining popularity and acceptance in the medtech industry, having been used to sanitize regenerative materials, biologic scaffolds and resorbable polymers ranging from poly-L-lactide and poly-D-lactide to L-lactide, DL-lactide and L-lactide/glycolide. Sutures could soon be added to the mix—last spring, NovaSterilis received a patent to remove toxic ethylene oxide (EO) residuals from absorbable sutures following sterilization. In working to obtain the patent, the company eliminated the need for EO sterilization altogether, achieving a Sterility Assurance Level of 10-6, with SCCO2 maintaining the sutures’ integrity.
“This new application can be applied across many classes of products that are sterilized with EO, potentially improving the performance of the product and outcomes of procedures utilizing these devices,” NovaSterilis President David Burns said upon the issuance of U.S. Patent No. 8658091. “We are confident this process can be expanded to a broad range of products and chemicals including, for example, the removal of residual solvents from pharmaceuticals.”
A legion of possibilities truly exists as the company attempts to branch into other sectors: Its SCCO2 technology already has infiltrated supranamics manufacturing, industrial degreasing, silicon wafer cleaning and textile dyeing. Vaccine/pharmaceutical purification and materials coatings are on the horizon as well.
The technology also is becoming more widespread within healthcare. More than a half-dozen U.S. tissue banks use SCCO2 to clean bone, tendon and other products; Australian Biotechnologies Pty. Ltd. received government approval in 2012 to market SCCO2-sterilized cortical and cancellous bone products; and a Dutch tissue processor/contract manufacturer is distributing SCCO2-treated allografts in Europe, India, China and Malaysia.
Indeed, the supercritical sterilization approach is gaining quite a toehold in the medtech arena. Yet many tissue banks are hesitant to invest in the new technology even though it can help suppliers significantly reduce costs by bringing the sterilization process in-house. In addition, the SCCO2 process—like gamma irradiation—can be performed while samples are sealed within their final packaging, reducing the opportunity for the reintroduction of contaminants.
Nevertheless, surgeons are optimistic about the future of supercritical sterilization. “I see the process becoming a standard in the industry,” one medtech executive speculated.
X-rays carry the same potential.
X-radiation is a viable alternative to hydrogen peroxide, steam and other traditional cleaning methods due to advancements in high-energy, high-power electron accelerators. Belgian firm Ion Beam Applications S.A. (IBA) is a pioneer in the field, having developed a particle accelerator in 1992 from a patented concept of the French Atomic Energy Commission. The company offers customers an X-ray sterilization solution called eXelis based on its high-power, high-energy Rhodotron accelerator, which provides one or several beam outputs from 2 to 10 MeV, with power ranging from 35 to 700 kW. IBA says eXelis helps improve the cost competitiveness of X-ray sterilization by reducing dose uniformity ratio (DUR) levels (the ratio between the maximum and minimum doses required to effectively process a product). Data supplied by IBA claim X-radiation can achieve a DUR of 1.25 for pallet loads of low-density materials compared with a DUR of 1.45 for the same low-density loads in a cobalt-60 gamma radiation facility.
X-ray sterilization offers several advantages over traditional methods like gamma radiation and ethylene oxide (EO). For starters, X-ray photons are more concentrated and directed than the widely scattered cobalt-60 gamma rays. X-ray systems also penetrate full pallets of product better than gamma and electron beam methods, hence their lower DURs. Pallets in X-ray facilities typically are irradiated from one side as they pass in front of a long, vertically-oriented target and irradiated on the opposite side to obtain a nearly uniform dose in the vertical direction.
The concentration of X-ray photons significantly reduces product exposure times, thereby minimizing or altogether eliminating damage to certain medical device materials. X-ray processing, in fact, can be used to chemically bond (cross-link) polymer chains together, effectually making plastics more resistant to stress and extreme conditions.
Despite its litany of benefits, however, X-ray sterilization is decidedly under-used due largely to a lack of capacity. The industry’s only large-scale medical device X-ray sterilization facility is owned by Synergy Health plc and located in Däniken, Switzerland. The British outsourcing provider of sterilization, laboratory, linen management, hospital and reusable surgical solution services inherited the plant with its 2012 purchase of Leoni Studer Hard AG for 47.6 million euros ($63.4 million). Featuring a 700kW Rhodotron electron beam accelerator, the facility can process up to 80,000 pallets annually.
“X-ray is an old technology, it’s been out for decades, but using it for sterilization purposes is a fairly new application. It’s the new kid on the block,” noted Mark Berger, Synergy’s business development manager. “Gamma radiation and EO processing have been around for 50 years and have built up such a reputation that they are the default methods. But there are other options out there.
Materials and coatings companies are partnering with sterilization providers and running tests to see what materials work best with the different [sterilization] processes. We have materials companies in the U.S. contacting our X-ray group in Switzerland to ask whether they can send the group materials so they can put together a white paper on the X-ray process and market the material. Companies are so used to using gamma or EO that they automatically think one of them will work for sterilization. Ninety-nine percent of the time they do work, but it’s not always the most cost-effective way to sterilize a product.”
Or the most suitable for the material.
Porous polyactone scaffolds, for example, best tolerate gas plasma processing while calcium phosphate bioceramics prefer EO (autoclaving significantly reduces its compressive strength). Gamma radiation is ideal for cross-linking ultra high molecular weight polyethylene (UHWPE) to reduce wear in acetabular hip liners, but the process can change the molecular weight of polylactic acid biodegradables (anchors for tendons and bone screws). Many newer orthopedic materials, in fact—drug/device combinations, hydrogels, polysaccharides and poly(amino acids)—are predisposed to room temperature sterilization processes.
Noxilizer Inc. provides such an alternative, disinfecting products at room temperature with minimal vacuum using nitrogen dioxide technology. The company’s NO2 sterilization process typically lasts less than three hours (including the aeration phase); vacuum can be used to provide a faster cycle for devices that can withstand pressure changes, but the NO2 gas readily diffuses the packaging when minimal vacuum is used to exchange process gases in the chamber.
One of the main advantages of NO2 technology is its compatibility with temperature-sensitive materials and drug-device combination products. Another major benefit of NO2 sterilization is its short aeration and cycle times. A routine cycle lasts 60-90 minutes (including 15 minutes for aeration), thus enabling a small sterilizer (about 360 liters of usable volume) to disinfect an entire pallet of product in an eight-hour shift and return it immediately to inventory. Such truncated cycle times are particularly important to orthopedic device manufacturers that customize implants for patients, as they generally are strapped for time to 3-D print, package and return products, industry experts claim.
Perhaps the greatest advantage of NO2 technology, however, is the potential for in-house processing, which can eliminate the transportation and inventory carrying costs typically incurred by higher volume manufacturers. NO2 gas is non-carcinogenic and non-flammable, making it considerably safer to set up in-house than gamma radiation, which requires a shielded storage room (wet or dry), a radiation shield (a thick concrete wall) surrounding the irradiation room, a control console room, and a product transport system. In addition, Noxilizer’s RTS 360 Industrial NO2 Sterilizer is designed to support lean manufacturing processes, and may be placed in line with assembly and packaging operations.
“Cost pressures within the orthopedics market are growing. This trend must be considered in orthopedic product development and manufacturing, and this is where sterilization can play a role,” said Opie, senior vice president of research and development at Noxilizer, a Baltimore, Md.-based seller of sterilization equipment and a contract NO2 sterilization provider to the medical device, biotechnology and pharmaceutical industries. “NO2 can help orthopedic manufacturers reduce turnaround of products and carrying costs by allowing small batches to be processed in-house. Costs per pallet are lower in-house than with a contract sterilizer. Technologies like gamma and EO are not easily brought in house—gamma requires building a radiation shield and EO has safety concerns. Outcomes-based reimbursement and other changes also will place cost pressures on hospitals performing surgical procedures. These cost pressures will impact the medical device supply chain, which includes the sterilization process. But these cost pressures and advancements like biodegradable implants and 3-D printing are giving NO2 an opportunity to grow. And that’ s good because there are lots of opportunities to grow—either from early adoption, companies looking to save money or those who truly want to change.”
Many customers, though, remain loyal to old favorites like gamma and EO. Sterigenics International LLC, for instance, spent more than $800 million last year beefing up its gamma capabilities, purchasing Canadian medical isotope/gamma technology provider Nordion Inc.; Mulberry, Fla.-based Food Technology Service Inc.; and Italian firm Gammarad. It also invested more than $10 million to expand its EO processing capacity in its Smyrna, Ga., Grand Prairie, Texas, and Petit-Rechain, Belgium, facilities.
For Sterigenics, the money is well spent. Despite their drawbacks, gamma radiation and ethylene oxide are still the preferred choice of medical device manufacturers, with companies nearly evenly split between both methods. Their appeal is multifaceted, though each has specific advantages over the other.
EO, for instance, is compatible with a wide range of materials and penetrates its target better than dry heat or steam. It also has the least impact on final material properties, which is particularly important for color-coded products.
“Manufacturers need to consider how the sterilization method will affect their product, such as color adonization or change to product composition,” explained Victoria Hughes, vice president of quality systems at Millstone Medical Outsourcing, a Fall River, Mass.-based medical device packaging provider. “Those colors usually indicate size. Companies need to consider product impact when weighing sterilization options.”
Ethylene oxide also is an effectual killer, eliminating all known viruses, bacteria, fungi and bacterial spores (typically the most sterilization-resistant type of microbe).
Gamma radiation is an efficient germ-slayer as well —the process disrupts pathogens’ DNA bond, preventing the cellular division necessary to sustain life. But the method leaves no harmful residue and is more permeable than EO, enabling products to be sterilized while sealed inside their final packaging (thus allowing device manufacturers to skip the post-sterilization drying and packaging step). And, since the radiation reaches the bulk inner material of the device, there is little, if any, risk of contamination within the body as the product degrades.
Gamma’s penetrability is specially appealing to artificial joint makers, who constantly are striving to build longer-lasting products. The process has no lasting impact on metals like titanium, stainless steel and chromium but it can change the molecular structure of polymers, leading to a better implant material for acetabular hip cups.
Radiation—either gamma or e-beam—is used in crosslinking, a process where long chains of polymers are linked together to increase its mass. Crosslinking also can make a polymer more scratch resistant and chemically immune, more responsive to higher temperatures, and improve shape memory retention (elastomers may be crosslinked to a slight degree to give them “memory”; they usually will return to their original shape after expansion).
“Crosslinking is well-known in the plastics industry to strengthen different types of polymers. The orthopedic industry is really looking to reduce the wear of the material,” said Scott Goetz, sales manager at Deerfield, Ill.-based Sterigenics, a contract sterilization provider to the medical device, pharmaceutical, food safety and high performance/specialty materials industries. “If you take the plastic or metal material and you rotate it on itself, eventually you’re going to wear it down and pieces of the plastic is going to go into the body and the body will reject it. The crosslinking actually takes the bonds of the ultra high molecular weight polymer and tightens it so you don’t have that wearing, or if you do, it takes a long longer to wear. The majority of ultra high molecular weight materials is crosslinked in gamma irradiation—it’s a more straightforward process. Currently, most of the implantable ultra high molecular weight polyethylene is radiation crosslinked, which reduces wear by 90 percent and will last the patient a lifetime. The latest trend in orthopedic implants is the introduction of Vitamin E-impregnated ultra high molecular weight polyethylene. The Vitamin E acts as an anti-oxidant in the polyethylene and therefore has less chance of rejection by the body, but at the same time it changes the process of radiation crosslinking that needs to be performed.”
* * *
Old habits die hard. Familiarity and tradition breed comfort, and comfort—in the board room, anyway—can beget institutional inertia. History is littered with the corpses of organizations that became a bit too comfortable with custom and resisted change (Radio Shack and Kodak are prime examples).
The medtech industry has never been too enamored of change. But companies increasingly are recognizing the benefits of innovative thinking as they attempt to keep pace with market changes. Nevertheless, there always will be some allegiance to routine and custom—even Jobs himself couldn’t escape the routine of daily product/management meetings and design lab time.
Sterilizers are striking a balance between the past and future, remaining loyal to gamma and EO methods for regulatory and manufacturing efficiency purposes, but exploring safer, less expensive alternatives. As Millstone’s Hughes noted: “In the knee, hip and shoulder areas, companies are not looking to get away from gamma or EO. They’ve been historically effective processes. But for newer devices that have electronics or are combination-type products, that’s where companies are looking into the newer methods.”