Ali Madani, CEO, Avicenne Medical05.29.18
Additive manufacturing is a disruptive fabrication process impacting an array of industry sectors, including healthcare. As such, members of this industry have made bold claims and predictions on the potential for this innovation. How much of this, however, has been based on a realistic view? That is likely a question to which a person would receive numerous responses, all with a different opinion being shared.
Attempting to cut through the hype to unveil the facts and offer a more reasonable perspective, the following frequently asked questions presentation has been provided. The inquiries cover many of the aspects often discussed when the topic of additive manufacturing is brought up with regard to its potential in the orthopedic industry.
Q: Is additive manufacturing truly on a pathway to revolutionize the fabrication of orthopedic products?
A: Additive manufacturing (AM) and related fabrication techniques have been revolutionizing the production of certain orthopedic products for a number of years. Orthopedic products produced using AM are primarily aimed at providing porous implants to enhance the rate of osseointegration following implantation of a prosthesis.
To provide some historical perspective, at the close of the 1990s, Zimmer used the IMPLEX Trabecular Tantalum technology, which they would later acquire in 2004. Zimmer began to produce and use this technology massively, leading the way to other powder-bed fusion technologies (EBM or SLM). Orthopedic companies like Lima, Beijing AK Medical, Adler Ortho, etc., bought several machines and, for over 10 years, learned the basics of this technology, adapting it to the orthopedics field and the fabrication of its products. Today, a firm like Lima owns more than 15 machines to manufacture its parts using AM.
Using this method, each firm produces thousands of parts per year—hip cup, knee, shoulder, cages, and dental implants. As a result, orthopedics, which is a very conservative field with an extremely long time to market, has changed its own habits, massively producing implants using AM.
Q: How involved in additive manufacturing are the major orthopedic device manufacturers?
A: Zimmer Biomet has chosen its own technology, which gives the same results as AM, but they are apparently also, to some extent, testing powder-bed fusion technologies.
Stryker is the other major company making significant investments into AM. They have announced their intention to invest more than $400 million into the acquisition of the necessary machines to produce this type of implant. Further, some of that funding will also be earmarked for the construction of one or several facilities that will focus on AM. Stryker’s strategy and investments in additive manufacturing constitute a major inflection point within the world of orthopedics. There was a “before” Stryker’s investments and there will be an “after” the investments, which were announced by the company in 2016.
Depuy Synthes, Smith & Nephew, and Medtronic are investing moderately in AM, often to manufacture plastic cut-blocks or certain prototypes, which, in the opinion of Avicenne Medical, are not priority applications of additive manufacturing.
Q: Is additive manufacturing technology mature enough today to meet market needs?
A: Research gathered as well as conducted by Avicenne Medical indicates that by the end of 2017, more than 300 additive manufacturing machines all over the world were producing orthopedic products. After 20 years of development and modifications, AM technology continues to evolve in manufacturing speed as well as output resolution. Companies like EOS, Arcam, Concept Laser, SLM Solutions, and several others are working diligently on the next generation of machines. Further, General Electric’s takeover of Arcam and Concept Laser—a total purchase price of more than $1.4 billion—also represents a crucial point for this industry, opening up new perspectives.
In addition, the supply of powder is another strategic factor that will need to be remedied by the emergence of new suppliers.
Q: How is additive manufacturing actually used in orthopedics and what are the prospects for the future?
A: Today in the reconstructive product market, there is a growing demand for additive manufacturing. For the spine, “standard” cages produced using AM are approaching mass market volumes. For certain complex implants (for example, expandable cages or complex customized implants), additive manufacturing facilitates the manufacturing process (compared to machining), and offers numerous competitive advantages. Nevertheless, Avicenne Medical believes forging, casting, and machining will continue to be the mass production processes for orthopedic products for several decades.
Q: Is additive manufacturing only adapted for internal manufacturing by orthopedic companies, or can subcontractors also exploit it for their customers?
A: Today, the most active orthopedic companies using AM are: Stryker, Lima, Adler, Beijing AK Medical, and Exactech, all who have invested significantly into this technology. In recent years, several larger contract manufacturers have begun to offer additive manufacturing services to orthopedic companies. Some multi-field contract manufacturers, like Eurocoating, now attribute more than 15 percent of their revenue to additive manufacturing.
There are several other possibilities as well. Industry demand for AM could result in the emergence of contract manufacturers who specialize in AM. Several attempts have already been made by certain companies that entered the market as specialist suppliers of unique additive manufacturing services.
Among the Top 100 historical orthopedic contract manufacturers, several dozen have acquired machines and offer additive manufacturing services to orthopedic companies.
Finally, let us not forget that even though companies currently employ the process internally, they could eventually outsource the method. Keep in mind that forging, machining, and other similar capabilities in the 1990s and 2000s were, for the most part, internal processes used by companies for their own products. Eventually, little by little, these “critical” processes were outsourced to contract manufacturers and supply partners. In some cases, the orthopedic manufacturer even sold the facilities housing these processes.
Over the long term, the additive manufacturing processes carried out inside orthopedic companies will continue to exist side by side with additive manufacturing services that are offered by contract manufacturers.
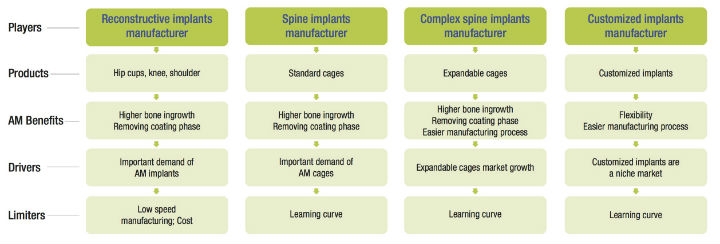
Source: Avicenne report 2018, Worldwide Additive Manufacturing & 3D Printing for Orthopedics 2016-2021 and Player Profiles. (Click image to view a larger version.)
Ali Madani has advised orthopedics firms and their contract manufacturers all over the world for more than 25 years on strategy, geographical expansion, innovations, and their production means and improvements thereto. Madani is the CEO of Avicenne Medical in Paris, France, and chairman of the annual IMPLANTS conference, which takes place in June.
Attempting to cut through the hype to unveil the facts and offer a more reasonable perspective, the following frequently asked questions presentation has been provided. The inquiries cover many of the aspects often discussed when the topic of additive manufacturing is brought up with regard to its potential in the orthopedic industry.
Q: Is additive manufacturing truly on a pathway to revolutionize the fabrication of orthopedic products?
A: Additive manufacturing (AM) and related fabrication techniques have been revolutionizing the production of certain orthopedic products for a number of years. Orthopedic products produced using AM are primarily aimed at providing porous implants to enhance the rate of osseointegration following implantation of a prosthesis.
To provide some historical perspective, at the close of the 1990s, Zimmer used the IMPLEX Trabecular Tantalum technology, which they would later acquire in 2004. Zimmer began to produce and use this technology massively, leading the way to other powder-bed fusion technologies (EBM or SLM). Orthopedic companies like Lima, Beijing AK Medical, Adler Ortho, etc., bought several machines and, for over 10 years, learned the basics of this technology, adapting it to the orthopedics field and the fabrication of its products. Today, a firm like Lima owns more than 15 machines to manufacture its parts using AM.
Using this method, each firm produces thousands of parts per year—hip cup, knee, shoulder, cages, and dental implants. As a result, orthopedics, which is a very conservative field with an extremely long time to market, has changed its own habits, massively producing implants using AM.
Q: How involved in additive manufacturing are the major orthopedic device manufacturers?
A: Zimmer Biomet has chosen its own technology, which gives the same results as AM, but they are apparently also, to some extent, testing powder-bed fusion technologies.
Stryker is the other major company making significant investments into AM. They have announced their intention to invest more than $400 million into the acquisition of the necessary machines to produce this type of implant. Further, some of that funding will also be earmarked for the construction of one or several facilities that will focus on AM. Stryker’s strategy and investments in additive manufacturing constitute a major inflection point within the world of orthopedics. There was a “before” Stryker’s investments and there will be an “after” the investments, which were announced by the company in 2016.
Depuy Synthes, Smith & Nephew, and Medtronic are investing moderately in AM, often to manufacture plastic cut-blocks or certain prototypes, which, in the opinion of Avicenne Medical, are not priority applications of additive manufacturing.
Q: Is additive manufacturing technology mature enough today to meet market needs?
A: Research gathered as well as conducted by Avicenne Medical indicates that by the end of 2017, more than 300 additive manufacturing machines all over the world were producing orthopedic products. After 20 years of development and modifications, AM technology continues to evolve in manufacturing speed as well as output resolution. Companies like EOS, Arcam, Concept Laser, SLM Solutions, and several others are working diligently on the next generation of machines. Further, General Electric’s takeover of Arcam and Concept Laser—a total purchase price of more than $1.4 billion—also represents a crucial point for this industry, opening up new perspectives.
In addition, the supply of powder is another strategic factor that will need to be remedied by the emergence of new suppliers.
Q: How is additive manufacturing actually used in orthopedics and what are the prospects for the future?
A: Today in the reconstructive product market, there is a growing demand for additive manufacturing. For the spine, “standard” cages produced using AM are approaching mass market volumes. For certain complex implants (for example, expandable cages or complex customized implants), additive manufacturing facilitates the manufacturing process (compared to machining), and offers numerous competitive advantages. Nevertheless, Avicenne Medical believes forging, casting, and machining will continue to be the mass production processes for orthopedic products for several decades.
Q: Is additive manufacturing only adapted for internal manufacturing by orthopedic companies, or can subcontractors also exploit it for their customers?
A: Today, the most active orthopedic companies using AM are: Stryker, Lima, Adler, Beijing AK Medical, and Exactech, all who have invested significantly into this technology. In recent years, several larger contract manufacturers have begun to offer additive manufacturing services to orthopedic companies. Some multi-field contract manufacturers, like Eurocoating, now attribute more than 15 percent of their revenue to additive manufacturing.
There are several other possibilities as well. Industry demand for AM could result in the emergence of contract manufacturers who specialize in AM. Several attempts have already been made by certain companies that entered the market as specialist suppliers of unique additive manufacturing services.
Among the Top 100 historical orthopedic contract manufacturers, several dozen have acquired machines and offer additive manufacturing services to orthopedic companies.
Finally, let us not forget that even though companies currently employ the process internally, they could eventually outsource the method. Keep in mind that forging, machining, and other similar capabilities in the 1990s and 2000s were, for the most part, internal processes used by companies for their own products. Eventually, little by little, these “critical” processes were outsourced to contract manufacturers and supply partners. In some cases, the orthopedic manufacturer even sold the facilities housing these processes.
Over the long term, the additive manufacturing processes carried out inside orthopedic companies will continue to exist side by side with additive manufacturing services that are offered by contract manufacturers.

Source: Avicenne report 2018, Worldwide Additive Manufacturing & 3D Printing for Orthopedics 2016-2021 and Player Profiles. (Click image to view a larger version.)
Ali Madani has advised orthopedics firms and their contract manufacturers all over the world for more than 25 years on strategy, geographical expansion, innovations, and their production means and improvements thereto. Madani is the CEO of Avicenne Medical in Paris, France, and chairman of the annual IMPLANTS conference, which takes place in June.