Sam Brusco, Associate Editor09.19.18
The earliest lathes can be traced back to about 1300 BC. These early devices required a two-person team—one who turned the wooden workpiece with rope, while the other cut shapes into the wood with sharp tools.
By the Middle Ages, pedal power supplanted hand-operated lathes. The first true machine lathe—a horizontal boring machine—was installed at the British Royal Arsenal in 1772. One of the crowning achievements of the Industrial Revolution, machining has been used extensively as a reliable manufacturing method since the 18th century.
Some aspects of machining equipment have endured, but Industrial Revolutionaries would gawk at what these devices are capable of today. Fully automated, CNC-powered machining centers can produce the work of a dozen or more men in a small fraction of the time, and can change out their tools should a drill bit break mid-operation. The relatively new process of electrical discharge machining (EDM) removes material from the workpiece via fast, nearly constant electrical current discharges between two electrodes. Machine enclosures used today tout soundproofing, temperature control, air-cleaning HVAC systems, and a bevy of other advanced features.
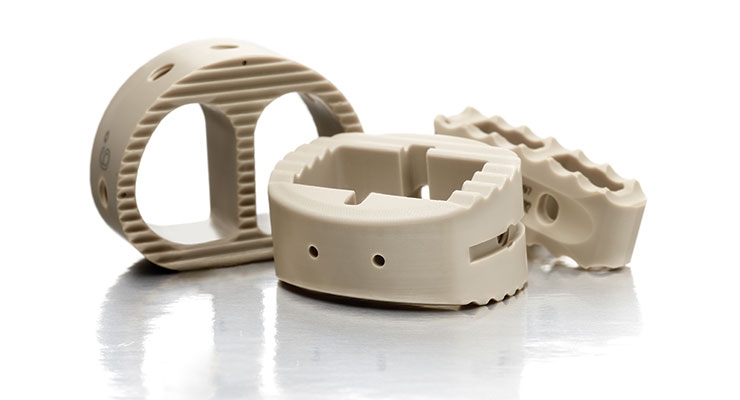
As orthopedic instruments and devices become more complex with tricky geometries and smaller configurations, precision machining must keep up in order to efficiently and reliably create these products. The manufacturing world may be enthusiastic about additive manufacturing’s potential, but the orthopedic industry still relies on proven machine, tooling, and cutting technologies.
On the OEM side of things, priority is placed on outpacing competitors on getting products to market, minimizing costs, and maintaining regulatory compliance. For machining vendors, this translates to rapid prototyping capabilities, effectively processing new materials, and working with increasingly tight tolerances, among other aspects.
“From the view point of the vendor, it begins with the basics—quality parts, on time delivery, and meeting the OEM’s perceived fair price,” said John MacDonald, president of AIP Precision Machining, a Daytona Beach, Fla.-based provider of precision machining services for the medical industry. “Of course, there are many more intangibles (which can be quite tangible) such as experience, ability to offer engineering solutions, and advice as well as trust.”
Because orthopedic instruments and devices are becoming more challenging to build as a result of trends toward personalized fits and minimally invasive procedures, orthopedic device manufacturers preach quality assurance. Precise dimensions, tight tolerances, surface finishes, and validated processes are a must have. In the orthopedic industry, quality control is literally a matter of life or death for machining vendors—if the critical dimensions don’t meet specifications, subpar product performance may harm patients.
“Orthopedic/medical device manufacturers desire a company with a robust quality management system capable of handling lot traceability and quality requirements within the multiple sizes and geometries of parts involved in an orthopedic device,” explained Mike Kaiser, vice president of business operations for Donatelle, a New Brighton, Minn.-based outsourcing services provider focused exclusively on the design, development, and manufacturing of medical devices.
“From a contract manufacturing standpoint, the ’Pick Two’ rule typically applies, which states that out of the options of good parts, made quickly, and at a cheap price, the customer can only pick two,” commented Thomas Fleckenstein, sales manager for Jarvis Surgical, a Westfield, Mass.-based manufacturer of precision components for the orthopedic industry. For example, you can have good parts made fast, but they won’t be cheap, or you can have cheap parts made really fast, but they won’t be good.”
Anyone in the business of developing medical devices—particularly Class III devices like orthopedic implants—adheres to a strict risk management program required by their governing regulatory body. ISO 13485:2016, published in early 2016, contains significant amendments to ensure companies’ quality management systems incorporate a risk-based approach. Naturally, this trickles down to machining vendors; orthopedic OEMs will only seek out those providers with a systematic application of management policies, procedures, and practices for analyzing, evaluating, controlling, and monitoring risk.
“Similar to other highly regulated markets, the medical device market is shifting to risk mitigation,” observed Randy Sible, general manager of Tegra Medical’s Hernando, Miss., location. Tegra Medical is an end-to-end (prototyping to full production) solutions provider for the medical device manufacturing industry. Its orthopedic focus includes spine, arthroscopy, trauma, total joint reconstruction, extremities, and sports medicine.
“Gone are the days of just making a part that meets the print,” he continued. “Now you have to prove your process is stable, identify and mitigate potential risks, and test worst-case scenarios to ensure the end product is not affected. In some cases, the paperwork takes longer to complete than the part. As a result, many small companies struggle with regulatory compliance. Meanwhile, when OEMs evaluate a vendor, they’re prioritizing the ability to adhere to quality requirements. Oftentimes, this is even more important than the ability to do the machining. OEMs are more apt to trust the contract manufacturers who have proven, solid experience.”
Sister publication Medical Product Outsourcing has made it clear that companies find it more efficient to focus on core competencies and new development projects and shift some processes to service providers or contract manufacturers (CMs). In the past, OEMs would turn to a number of suppliers or CMs to lessen the load, but this is changing as well. Because of the aforementioned stress on risk management, OEMs are no longer deeming it prudent to have a long list of suppliers and CMs. Rather, they seek out partners who are more vertically integrated, with a number of value-added services so they can do their shopping in one place and reduce supply chain risk.
For machining vendors, this means having capabilities in several types of machining depending on the project’s need—including EDM, laser machining, computer numerical control (CNC) machining, and others. It also means machining vendors are no longer simply making and validating the parts. They are also expected to provide engineering support, cleanroom assembly, packaging capabilities, and whatever services are possible to prevent a large, potentially risky supplier base.
“Orthopedic and medical device OEMs trend toward reducing the number of approved suppliers and dedicate a considerable amount of resources in identification, qualification, and the ongoing audit activities of chosen suppliers,” stated Patrick Pickerell, president of Peridot Corp., a Pleasanton, Calif.-based provider of medical device component machining and cleanroom assembly. “It is more sensible to invest this effort and expense in a full-service supplier rather than having to qualify multiple suppliers on a device or implant program. We feature EDM, Laser, CNC machining, metal-forming, cleanroom assembly, and packaging under one roof.”
“Medical device manufacturers seek us out for our engineering and Class III implantable experience, full-service capabilities, and vertical integration that allows us to take on a larger part of their supply chain within a one-stop location,” noted Kaiser. “We can offer solutions in metal and plastic machining; and as the volumes increase, we can provide options in injection molding and Micro-MIM to provide the best overall cost and quality solution.”
However, vertical integration isn’t necessarily the only option for machining vendors to become more attractive to orthopedic or medical device manufacturers. As any entrepreneur will attest to, finding an underserved niche and developing expertise there is an extremely viable way to ensure continued business. Take, for example, the machining of polymer components.
When design engineers require a custom-machined component, the instinct is to select a metallic material. And while it’s true that load-bearing orthopedic implants still use titanium to a large degree, certain polymers are quite prevalent—especially since metal-on-metal orthopedic implants fell out of favor due to their health concerns. Artificial hip joints routinely feature metal-on-polyethylene construction, and polyether ether ketone (PEEK) spine implants are becoming more mainstream.
Just because a vendor has expertise in machining metals doesn’t mean it will easily translate into machining polymers, however. Plastics exhibit a wide range of mechanical and thermal properties that result in varying behavior when machined; it’s critical to have a strong knowledge of polymer structure and material properties when machining it. For example, some plastics may be more brittle, while others cut similarly to metal.
“We are a niche player in the machining world with over 36 years of experience machining plastic and composite materials,” said MacDonald. “We do not machine metals, and the machined metal market is huge compared to the machined polymer market. OEMs come to us when they venture into or have a need for mission critical plastic parts. Maintaining and achieving tight tolerances in plastic parts is a totally different world than doing the same in metal. It isn’t any more or less difficult, just different. It is sort of the same thought process as not using your cardiologist for an orthopedic injury. Both are very capable doctors, but each has a concentration or specialty.”
Advancing Both Machine and Man
Improving machining technology and automation is a continual process, supported by the steady flow of announcements by equipment manufacturers regarding new machines that can extend tool life, reduce costs, and improve the manufacturing process. The latest machines and tooling capabilities enable contract manufacturers to offer an expanded range of services for various design and production stages—all the way from concept evaluation through full production.
Many machine manufacturers now offer full 5-axis CNC machines intended specifically for orthopedic component manufacturing, as well as the requirements those components pose for their manufacture. CNC Swiss machines—some as high as 13-axis—are ideal for the complex part geometries necessary for orthopedic device manufacturing. These machines allow incredibly precise movements that can be programmed for optimal speeds and feeds, which enables faster cycle times.
Reduced pricing and speedier delivery requirements demanded by orthopedic device manufacturers are also pushing machining vendors to invest heavily in automation. Automation increases both efficiency and productivity, and allows more unattended machine time so capacity can be maximized. To provide the quick turnaround times demanded by manufacturers, machining providers find new ways to boost the automation of their machines and facilities to augment efficiency and throughput.
“We are implementing new technologies, such as robotic and palletized loading systems, to increase efficiencies in all areas of the manufacturing process,” said Ryan Thornburgh, business development manager of Precision Medical Technologies, a Warsaw, Ind.-based contract manufacturer of orthopedic and medical instruments and implants. “We are currently utilizing robotic finishing and inspection equipment to minimize variations in the manufacturing process, as well as drive high-quality performance in all facilities.”
Automated inspection equipment also monitors every phase of the machining process to verify its precision performance. In the past, quality personnel had to take the components off the machine, travel to the quality control room to perform coordinate measuring machine inspection, conclude the part meets specifications, and then repeat. Automated CNC inspection systems can automatically and directly inspect parts on the machine after machining is completed, saving time and reducing the risk of error associated with repeated loading and centering.
“Automation is key to two very important aspects in our industry,” declared John Ruggieri, vice president of engineering and business development for ARCH Medical Solutions, Seabrook, a Seabrook, N.H.-based manufacturer of highly-engineered, complex, precision-machined medical instruments and implants across multiple orthopedic product groups such as spine, knee, extremities, and trauma. “First and foremost, repeatable process capability is vital for reducing risk for our customers. After first articles are approved, the process must be stable enough to produce at large scales. Changes after this point are costly, and in most cases, unsupported by the industry due to the regulatory steps needed to implement improvements. Second, it is important for manufacturers to maintain their competitiveness and reduce labor costs. Minimizing set-ups, reducing operator interventions, and keeping machines producing are an absolute requirement for delivering value.”
Another popular method to ramp up production speed and reduce the number of steps to machine a part or product is “hybrid” laser-Swiss equipment. Hybrid equipment is named as such because it merges Swiss turning, laser cutting, and laser welding steps into one setup, thereby allowing parts to be made at a single station.
“There have been some fascinating advances in machining that combine processes in one work center—such as onboard cutting lasers—incorporated into CNC Swiss machines allowing for both turning and laser cutting in one work center,” explained Pickerell.
Another fairly recent trend involves merging multi-axis CNC machining with laser sintering, in a process referred to as hybrid manufacturing. This involves the combination of additive and subtractive manufacturing in a single system. For this process, the machine pretty much creates its own near net part geometry with the on-board laser sintering. The part can then be precision machined with the CNC part of the machine into dimensional tolerance. However, the process is still somewhat in its infancy.
“Advances continue to be made in additive processes like DMLS (Direct Metal Laser Sintering) with part resolution getting better, but still not close to machining tolerances,” Pickerell continued. “Once this technology matures and comes down in price, I predict much wider adoption and utilization.”
Machining vendors can further boost efficiencies and cut costs by developing their own custom machining tools. It can help to open up capabilities in their CNC machines and ensure high-quality tools for the process, leading to speedier production and improved outcomes for complex orthopedic projects.
“Custom machine tools allow integrating multiple manufacturing processes that are performed separately which, in turn, helps improve quality and efficiency of the manufacturing process,” stated Raghu Vadlamudi, Donatelle’s chief research and technology officer. “Examples include inserting a radio opaque marker into a machined orthopedic cage, and integrating laser welding and cutting with conventional machine tools.”
Until additive manufacturing gains greater acceptance and ensures repeatability, CNC machining combined with lasers, welding, and cutting capabilities will be orthopedic device manufacturers’ go-to process. But orthopedic devices themselves will continue to become more complex as customized fits and minimally invasive procedures continue to proliferate. It is then machining vendors’ responsibility to offer an equally customized approach. In-house custom tools further reduce order times, accelerating turnaround time and reducing the customer’s all-important time to market.
“Custom tools are often used to machine complex and compound surfaces that would otherwise require timelier and, therefore, more expensive operations (sculpting, electrical discharge machining, etc.),” commented Fleckenstein. “In my experience, it is not that standard tooling is incapable of machining the same geometries and tolerances, but that they take longer and typically require multiple setups/operations.”
“This is by no means intended to be a failsafe or one-size-fits-all statement,” he further advised. “To borrow an axiom, there is more than one way to skin a cat. I could ask 10 machinists and 10 programmers in our shop how to machine any given part and I will most likely get 20 different answers. Still, given past experience, more often than not, most of them would prefer to use a custom slitter saw or form tool in five axes over a ball end mill using three axes.”
An important point to note is while the machines themselves are capable of amazing things, as of 2018 they still require an operator. A specialized skill set is required to machine orthopedic devices, instruments, and components, and the more complex orthopedic technology becomes with respect to complex geometries and new materials, the more machinists must learn to successfully hone their craft.
“Continuing advances in manufacturing technology will affect the skill set that is required,” predicted Sible. “More sophisticated machines will be easier to use during the production process. The manufacturing process will, however, require more up-front error proofing and risk mitigation before getting to the machining stage. We can expect more focus on engineering and the entire process, with the goal of creating more consistent, predictable machining.”
Machining vendors naturally place a lot of emphasis on their training programs in order to properly educate craftsmen on how to operate new, complex machinery.
“We expect machinists to know inspection tools and robotic inspection equipment, as well as the appropriate applications of those tools,” said Thornburgh. “We also train each machinist in blueprint reading with knowledge of geometric dimensioning and tolerance. We believe these skill sets are vital to the future of orthopedic device manufacturing, and we expect our machinists to be well-adept.”
The medical device industry continues to crave miniaturization, and the orthopedic segment of course follows suit. Coupled with new, biocompatible materials being introduced into orthopedic devices, machinists must be cognizant of the technological trends and accompanying scientific principles to remain current.
“We are working on a process to shrink the height of our staff to one-half of actual human size to position us for servicing the smaller parts we expect to see,” joked Pickerell. “All kidding aside, the machining challenges we face going forward are substantial. The properties of high-performance biocompatible materials are often at odds with the ease of machining, so we will have to innovate and get even smarter to survive.”
The medical device industry has recently had some trouble recruiting capable young machinists. The learning curve to become a precision machinist can be laborious, and it doesn’t help matters that the current entry-level workforce grew up in a time where information is readily available, often resulting in a lack of patience or desire for training. The problem also arises from the mentality that a career in manufacturing isn’t viewed as alluring or gratifying as compared to “cleaner” white-collar jobs.
“Machining always has been, and always will be, about finding talented craftsmen,” concluded MacDonald. “If I have learned anything in my 27 years working in and around machine shops, it’s that it is all about the people. However, the trades continue to struggle to attract new talent. The men and women who machine custom tight tolerance parts for a living—especially in a true job shop environment—are more artists than employees. Creative thinking mixed with technical know-how and good old farm hand solutions to new challenges provides for a very rewarding and satisfying career. The best way to learn such a trade is by working in an apprentice type capacity. It does not come quickly, but the knowledge gained by working with and around experienced machinists forms the basis of learning this trade. Machine technology has advanced exponentially and has allowed companies like ours to deliver parts we would have been unable to with older technology. But the machines and technology are just a nice pile of metal, wires, and circuitry without the hands and mind of a creative programmer/machinist.”
By the Middle Ages, pedal power supplanted hand-operated lathes. The first true machine lathe—a horizontal boring machine—was installed at the British Royal Arsenal in 1772. One of the crowning achievements of the Industrial Revolution, machining has been used extensively as a reliable manufacturing method since the 18th century.
Some aspects of machining equipment have endured, but Industrial Revolutionaries would gawk at what these devices are capable of today. Fully automated, CNC-powered machining centers can produce the work of a dozen or more men in a small fraction of the time, and can change out their tools should a drill bit break mid-operation. The relatively new process of electrical discharge machining (EDM) removes material from the workpiece via fast, nearly constant electrical current discharges between two electrodes. Machine enclosures used today tout soundproofing, temperature control, air-cleaning HVAC systems, and a bevy of other advanced features.
As orthopedic instruments and devices become more complex with tricky geometries and smaller configurations, precision machining must keep up in order to efficiently and reliably create these products. The manufacturing world may be enthusiastic about additive manufacturing’s potential, but the orthopedic industry still relies on proven machine, tooling, and cutting technologies.
On the OEM side of things, priority is placed on outpacing competitors on getting products to market, minimizing costs, and maintaining regulatory compliance. For machining vendors, this translates to rapid prototyping capabilities, effectively processing new materials, and working with increasingly tight tolerances, among other aspects.
“From the view point of the vendor, it begins with the basics—quality parts, on time delivery, and meeting the OEM’s perceived fair price,” said John MacDonald, president of AIP Precision Machining, a Daytona Beach, Fla.-based provider of precision machining services for the medical industry. “Of course, there are many more intangibles (which can be quite tangible) such as experience, ability to offer engineering solutions, and advice as well as trust.”
Because orthopedic instruments and devices are becoming more challenging to build as a result of trends toward personalized fits and minimally invasive procedures, orthopedic device manufacturers preach quality assurance. Precise dimensions, tight tolerances, surface finishes, and validated processes are a must have. In the orthopedic industry, quality control is literally a matter of life or death for machining vendors—if the critical dimensions don’t meet specifications, subpar product performance may harm patients.
“Orthopedic/medical device manufacturers desire a company with a robust quality management system capable of handling lot traceability and quality requirements within the multiple sizes and geometries of parts involved in an orthopedic device,” explained Mike Kaiser, vice president of business operations for Donatelle, a New Brighton, Minn.-based outsourcing services provider focused exclusively on the design, development, and manufacturing of medical devices.
“From a contract manufacturing standpoint, the ’Pick Two’ rule typically applies, which states that out of the options of good parts, made quickly, and at a cheap price, the customer can only pick two,” commented Thomas Fleckenstein, sales manager for Jarvis Surgical, a Westfield, Mass.-based manufacturer of precision components for the orthopedic industry. For example, you can have good parts made fast, but they won’t be cheap, or you can have cheap parts made really fast, but they won’t be good.”
Anyone in the business of developing medical devices—particularly Class III devices like orthopedic implants—adheres to a strict risk management program required by their governing regulatory body. ISO 13485:2016, published in early 2016, contains significant amendments to ensure companies’ quality management systems incorporate a risk-based approach. Naturally, this trickles down to machining vendors; orthopedic OEMs will only seek out those providers with a systematic application of management policies, procedures, and practices for analyzing, evaluating, controlling, and monitoring risk.
“Similar to other highly regulated markets, the medical device market is shifting to risk mitigation,” observed Randy Sible, general manager of Tegra Medical’s Hernando, Miss., location. Tegra Medical is an end-to-end (prototyping to full production) solutions provider for the medical device manufacturing industry. Its orthopedic focus includes spine, arthroscopy, trauma, total joint reconstruction, extremities, and sports medicine.
“Gone are the days of just making a part that meets the print,” he continued. “Now you have to prove your process is stable, identify and mitigate potential risks, and test worst-case scenarios to ensure the end product is not affected. In some cases, the paperwork takes longer to complete than the part. As a result, many small companies struggle with regulatory compliance. Meanwhile, when OEMs evaluate a vendor, they’re prioritizing the ability to adhere to quality requirements. Oftentimes, this is even more important than the ability to do the machining. OEMs are more apt to trust the contract manufacturers who have proven, solid experience.”
Sister publication Medical Product Outsourcing has made it clear that companies find it more efficient to focus on core competencies and new development projects and shift some processes to service providers or contract manufacturers (CMs). In the past, OEMs would turn to a number of suppliers or CMs to lessen the load, but this is changing as well. Because of the aforementioned stress on risk management, OEMs are no longer deeming it prudent to have a long list of suppliers and CMs. Rather, they seek out partners who are more vertically integrated, with a number of value-added services so they can do their shopping in one place and reduce supply chain risk.
For machining vendors, this means having capabilities in several types of machining depending on the project’s need—including EDM, laser machining, computer numerical control (CNC) machining, and others. It also means machining vendors are no longer simply making and validating the parts. They are also expected to provide engineering support, cleanroom assembly, packaging capabilities, and whatever services are possible to prevent a large, potentially risky supplier base.
“Orthopedic and medical device OEMs trend toward reducing the number of approved suppliers and dedicate a considerable amount of resources in identification, qualification, and the ongoing audit activities of chosen suppliers,” stated Patrick Pickerell, president of Peridot Corp., a Pleasanton, Calif.-based provider of medical device component machining and cleanroom assembly. “It is more sensible to invest this effort and expense in a full-service supplier rather than having to qualify multiple suppliers on a device or implant program. We feature EDM, Laser, CNC machining, metal-forming, cleanroom assembly, and packaging under one roof.”
“Medical device manufacturers seek us out for our engineering and Class III implantable experience, full-service capabilities, and vertical integration that allows us to take on a larger part of their supply chain within a one-stop location,” noted Kaiser. “We can offer solutions in metal and plastic machining; and as the volumes increase, we can provide options in injection molding and Micro-MIM to provide the best overall cost and quality solution.”
However, vertical integration isn’t necessarily the only option for machining vendors to become more attractive to orthopedic or medical device manufacturers. As any entrepreneur will attest to, finding an underserved niche and developing expertise there is an extremely viable way to ensure continued business. Take, for example, the machining of polymer components.
When design engineers require a custom-machined component, the instinct is to select a metallic material. And while it’s true that load-bearing orthopedic implants still use titanium to a large degree, certain polymers are quite prevalent—especially since metal-on-metal orthopedic implants fell out of favor due to their health concerns. Artificial hip joints routinely feature metal-on-polyethylene construction, and polyether ether ketone (PEEK) spine implants are becoming more mainstream.
Just because a vendor has expertise in machining metals doesn’t mean it will easily translate into machining polymers, however. Plastics exhibit a wide range of mechanical and thermal properties that result in varying behavior when machined; it’s critical to have a strong knowledge of polymer structure and material properties when machining it. For example, some plastics may be more brittle, while others cut similarly to metal.
“We are a niche player in the machining world with over 36 years of experience machining plastic and composite materials,” said MacDonald. “We do not machine metals, and the machined metal market is huge compared to the machined polymer market. OEMs come to us when they venture into or have a need for mission critical plastic parts. Maintaining and achieving tight tolerances in plastic parts is a totally different world than doing the same in metal. It isn’t any more or less difficult, just different. It is sort of the same thought process as not using your cardiologist for an orthopedic injury. Both are very capable doctors, but each has a concentration or specialty.”
Advancing Both Machine and Man
Improving machining technology and automation is a continual process, supported by the steady flow of announcements by equipment manufacturers regarding new machines that can extend tool life, reduce costs, and improve the manufacturing process. The latest machines and tooling capabilities enable contract manufacturers to offer an expanded range of services for various design and production stages—all the way from concept evaluation through full production.
Many machine manufacturers now offer full 5-axis CNC machines intended specifically for orthopedic component manufacturing, as well as the requirements those components pose for their manufacture. CNC Swiss machines—some as high as 13-axis—are ideal for the complex part geometries necessary for orthopedic device manufacturing. These machines allow incredibly precise movements that can be programmed for optimal speeds and feeds, which enables faster cycle times.
Reduced pricing and speedier delivery requirements demanded by orthopedic device manufacturers are also pushing machining vendors to invest heavily in automation. Automation increases both efficiency and productivity, and allows more unattended machine time so capacity can be maximized. To provide the quick turnaround times demanded by manufacturers, machining providers find new ways to boost the automation of their machines and facilities to augment efficiency and throughput.
“We are implementing new technologies, such as robotic and palletized loading systems, to increase efficiencies in all areas of the manufacturing process,” said Ryan Thornburgh, business development manager of Precision Medical Technologies, a Warsaw, Ind.-based contract manufacturer of orthopedic and medical instruments and implants. “We are currently utilizing robotic finishing and inspection equipment to minimize variations in the manufacturing process, as well as drive high-quality performance in all facilities.”
Automated inspection equipment also monitors every phase of the machining process to verify its precision performance. In the past, quality personnel had to take the components off the machine, travel to the quality control room to perform coordinate measuring machine inspection, conclude the part meets specifications, and then repeat. Automated CNC inspection systems can automatically and directly inspect parts on the machine after machining is completed, saving time and reducing the risk of error associated with repeated loading and centering.
“Automation is key to two very important aspects in our industry,” declared John Ruggieri, vice president of engineering and business development for ARCH Medical Solutions, Seabrook, a Seabrook, N.H.-based manufacturer of highly-engineered, complex, precision-machined medical instruments and implants across multiple orthopedic product groups such as spine, knee, extremities, and trauma. “First and foremost, repeatable process capability is vital for reducing risk for our customers. After first articles are approved, the process must be stable enough to produce at large scales. Changes after this point are costly, and in most cases, unsupported by the industry due to the regulatory steps needed to implement improvements. Second, it is important for manufacturers to maintain their competitiveness and reduce labor costs. Minimizing set-ups, reducing operator interventions, and keeping machines producing are an absolute requirement for delivering value.”
Another popular method to ramp up production speed and reduce the number of steps to machine a part or product is “hybrid” laser-Swiss equipment. Hybrid equipment is named as such because it merges Swiss turning, laser cutting, and laser welding steps into one setup, thereby allowing parts to be made at a single station.
“There have been some fascinating advances in machining that combine processes in one work center—such as onboard cutting lasers—incorporated into CNC Swiss machines allowing for both turning and laser cutting in one work center,” explained Pickerell.
Another fairly recent trend involves merging multi-axis CNC machining with laser sintering, in a process referred to as hybrid manufacturing. This involves the combination of additive and subtractive manufacturing in a single system. For this process, the machine pretty much creates its own near net part geometry with the on-board laser sintering. The part can then be precision machined with the CNC part of the machine into dimensional tolerance. However, the process is still somewhat in its infancy.
“Advances continue to be made in additive processes like DMLS (Direct Metal Laser Sintering) with part resolution getting better, but still not close to machining tolerances,” Pickerell continued. “Once this technology matures and comes down in price, I predict much wider adoption and utilization.”
Machining vendors can further boost efficiencies and cut costs by developing their own custom machining tools. It can help to open up capabilities in their CNC machines and ensure high-quality tools for the process, leading to speedier production and improved outcomes for complex orthopedic projects.
“Custom machine tools allow integrating multiple manufacturing processes that are performed separately which, in turn, helps improve quality and efficiency of the manufacturing process,” stated Raghu Vadlamudi, Donatelle’s chief research and technology officer. “Examples include inserting a radio opaque marker into a machined orthopedic cage, and integrating laser welding and cutting with conventional machine tools.”
Until additive manufacturing gains greater acceptance and ensures repeatability, CNC machining combined with lasers, welding, and cutting capabilities will be orthopedic device manufacturers’ go-to process. But orthopedic devices themselves will continue to become more complex as customized fits and minimally invasive procedures continue to proliferate. It is then machining vendors’ responsibility to offer an equally customized approach. In-house custom tools further reduce order times, accelerating turnaround time and reducing the customer’s all-important time to market.
“Custom tools are often used to machine complex and compound surfaces that would otherwise require timelier and, therefore, more expensive operations (sculpting, electrical discharge machining, etc.),” commented Fleckenstein. “In my experience, it is not that standard tooling is incapable of machining the same geometries and tolerances, but that they take longer and typically require multiple setups/operations.”
“This is by no means intended to be a failsafe or one-size-fits-all statement,” he further advised. “To borrow an axiom, there is more than one way to skin a cat. I could ask 10 machinists and 10 programmers in our shop how to machine any given part and I will most likely get 20 different answers. Still, given past experience, more often than not, most of them would prefer to use a custom slitter saw or form tool in five axes over a ball end mill using three axes.”
An important point to note is while the machines themselves are capable of amazing things, as of 2018 they still require an operator. A specialized skill set is required to machine orthopedic devices, instruments, and components, and the more complex orthopedic technology becomes with respect to complex geometries and new materials, the more machinists must learn to successfully hone their craft.
“Continuing advances in manufacturing technology will affect the skill set that is required,” predicted Sible. “More sophisticated machines will be easier to use during the production process. The manufacturing process will, however, require more up-front error proofing and risk mitigation before getting to the machining stage. We can expect more focus on engineering and the entire process, with the goal of creating more consistent, predictable machining.”
Machining vendors naturally place a lot of emphasis on their training programs in order to properly educate craftsmen on how to operate new, complex machinery.
“We expect machinists to know inspection tools and robotic inspection equipment, as well as the appropriate applications of those tools,” said Thornburgh. “We also train each machinist in blueprint reading with knowledge of geometric dimensioning and tolerance. We believe these skill sets are vital to the future of orthopedic device manufacturing, and we expect our machinists to be well-adept.”
The medical device industry continues to crave miniaturization, and the orthopedic segment of course follows suit. Coupled with new, biocompatible materials being introduced into orthopedic devices, machinists must be cognizant of the technological trends and accompanying scientific principles to remain current.
“We are working on a process to shrink the height of our staff to one-half of actual human size to position us for servicing the smaller parts we expect to see,” joked Pickerell. “All kidding aside, the machining challenges we face going forward are substantial. The properties of high-performance biocompatible materials are often at odds with the ease of machining, so we will have to innovate and get even smarter to survive.”
The medical device industry has recently had some trouble recruiting capable young machinists. The learning curve to become a precision machinist can be laborious, and it doesn’t help matters that the current entry-level workforce grew up in a time where information is readily available, often resulting in a lack of patience or desire for training. The problem also arises from the mentality that a career in manufacturing isn’t viewed as alluring or gratifying as compared to “cleaner” white-collar jobs.
“Machining always has been, and always will be, about finding talented craftsmen,” concluded MacDonald. “If I have learned anything in my 27 years working in and around machine shops, it’s that it is all about the people. However, the trades continue to struggle to attract new talent. The men and women who machine custom tight tolerance parts for a living—especially in a true job shop environment—are more artists than employees. Creative thinking mixed with technical know-how and good old farm hand solutions to new challenges provides for a very rewarding and satisfying career. The best way to learn such a trade is by working in an apprentice type capacity. It does not come quickly, but the knowledge gained by working with and around experienced machinists forms the basis of learning this trade. Machine technology has advanced exponentially and has allowed companies like ours to deliver parts we would have been unable to with older technology. But the machines and technology are just a nice pile of metal, wires, and circuitry without the hands and mind of a creative programmer/machinist.”