Sam Brusco , Associate Editor11.30.18
Accidents happen. And when they do, they will most likely involve damage to the musculoskeletal system. In fact, according to the American Academy of Orthopaedic Surgeons, more than three out of every five injuries that occur annually in the United States are to the musculoskeletal system.
It’s no surprise, then, that the trauma product market continues to thrive simply because three out of five people involved in a physical mishap will require some degree of orthopedic care. Some of those will need technology to assist in their recovery, fueling the trauma products market that B2B research firm MarketsandMarkets expects to grow 7.1 percent to $8.43 billion by the year 2022. This growth was attributed to a high prevalence of bone degenerative diseases, increasing incidence of road accidents and falls, and rising amounts of sports injuries.
Traditional technologies used to treat traumatic orthopedic injuries include internal fixators (plates, screws, nails, pins, wires, and staples), external fixators (unilateral and bilateral, circular, and hybrid), and a variety of other diverse trauma products. Orthopedic surgeons typically prefer internal fixators because they are used for fractures that can only be surgically repaired (i.e., unstable, pathological, and multiple fractures). External fixators are used in cases of open fractures, infected fractures, closed fractures, burns, and damage control orthopedics. Hospitals and trauma centers as well as ambulatory surgical centers (ASCs) are the primary end-users of these types of technologies.
The market is highly competitive, with both small and large players seeking a share. This article will explore three companies taking an innovative approach to developing technologies that assist patients when accidents happen.
Streamlining Efficiency and Value in the OR
Operating rooms are one of the costliest areas for most hospitals. Hospitals face a range of mounting financial pressures, so many are reexamining OR operations for avoidable costs. And as care and reimbursement moves closer to value-based models, orthopedic surgeons are continuing to seek out tools and resources to provide patients with the best care while lowering the cost of delivery.
Value-based care could perhaps have the largest effect on trauma and fracture patients. This type of care is emergent or urgent, and as a result, can have a significant number of unfunded or underfunded patients. However, the most expensive factor involved in fracture care surgery is typically the cost of the implant. In fact, many uninsured or underfunded patients are unable to have surgeries performed at outpatient centers because of prohibitive implant costs. With rising healthcare costs and decreasing physician and hospital reimbursement, this model isn’t sustainable in the long-term.
To gain more insight on this, ODT spoke with Steve Lichtenthal, vice president of business development for The Orthopaedic Implant Company (OIC), a Reno, Nev.-based orthopedic trauma implant manufacturer.
Brusco: What is unique about The Orthopaedic Implant Company’s orthopedic trauma portfolio?
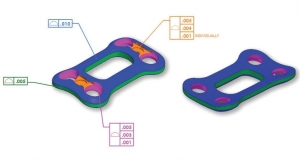
Lichtenthal:Our orthopedic trauma portfolio was designed to bring efficiency and value in the OR. Oftentimes streamlined manufacturability and operating room efficiency is not considered a factor in orthopedic device design, resulting in the devices being far more expensive than their functional value would suggest. Our trauma portfolio is designed to be simple and straightforward. By and large, conventional vendors design implants and systems to validate the need for representation in the OR and hospital supply chain. Our engineering team is tasked with making things as intuitive and predictable as possible. This approach mitigates the need for vendor representation in the OR and in the hospital’s supply chain as well.
Brusco: OIC states that it is “…driven to change the way implants are manufactured and priced.” Can you elaborate on that? How does it apply to your trauma portfolio?
Lichtenthal: The measures that we have taken to create our unique model of delivery makes it possible to accomplish our primary goal—to make value-based care for patients and hospitals a reality. Our model halves implant spending. It is important to note that studies have proven our implants to be clinically equivalent to comparable, premium brand implants on the market today. Furthermore, these studies have documented and proven the value as well. The healthcare landscape is quickly moving from fee-for-service to value-based care models. We have committed to being part of the solution to help providers maintain standards in clinical outcomes, while helping them best the challenges value-based care presents.
Brusco: What are some future goals for the company’s trauma portfolio? (Next-gen products, new indications, etc.)
Lichtenthal: We have been very pleased with the adoption of our model. From rural hospitals to level 1 academic centers in urban areas, our value proposition has been proven to be a growing mainstay in the market. As our relationships with surgeons and facilities strengthen, we get incredible insight and feedback on how we should expand our offering. While we continue to add to our trauma portfolio, next year the market will see our first implant that goes beyond orthopedic trauma. Largely untouched by high-value vendors, this area of orthopedics is clamoring for savings and the impact is going to be incredible.
Brusco: Is there anything else you’d like to say regarding orthopedic trauma solutions or the orthopedic trauma market?
Lichtenthal: The market is quickly changing to reward value. That’s a first for orthopedic implants and the broader healthcare market as well. All of the vendors understand it, although few are willing to step up and take the reins. Most vendors’ strategies revolve around price preservation, betting on the rift between surgeons’ and hospitals’ goals and motivations. The reality is that rift is narrowing quickly and high-value implants are just an example of a bridge that leads to success for everyone—most importantly, the patient.
Transforming High-Energy Trauma Care
A condition called compartment syndrome can occur when muscle pressure builds to dangerous levels. This pressure can decrease blood flow, preventing oxygen from reaching nerve and muscle cells. It can either be acute or chronic, and acute compartment syndrome (ACS) is usually caused by a severe injury—a fracture, badly bruised muscle, or crush injury, for example. Left untreated, ACS can lead to permanent muscle damage. The problem is, diagnosing ACS relies on subjective pain measurements and often inaccurate sensing technology.
To further explore this, ODT spoke with Dr. Edward J. Harvey H.BSc, MDCM, MSc, FRCSC and co-founder of MY01, which created a digital medical device to diagnose ACS. Nxtsens Microsystems, which helped to develop MY01, specializes in sensor technology. Nxtsens is on a mission to empower healthcare professionals with the ability to preempt severe medical conditions, improving patient outcomes. Based in Montreal, Quebec, their team specializes in next-generation cloud-enabled MEMS technology.
Brusco: How does MY01 work? How does it differ from other solutions to diagnose ACS?
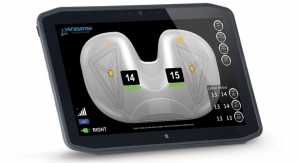
Dr. Harvey: MY01 is a digitally connected medical device that aids in the diagnosis of acute compartment syndrome (ACS). Its insertion mechanism allows easy, accurate placement of a small pressure sensor inside a patient's muscle compartment. The sensor connects to a body-worn display screen through a biocompatible lead-wire, and continuously outputs accurate pressure values while resting on a patient’s skin. MY01 is accompanied by a mobile application that wirelessly connects to the medical device via Bluetooth. Our app transfers pressure data to hospital cloud systems, enabling remote data display, and also allows clinicians to log patient pain scores, notes, and images.
Currently, ACS is diagnosed based on subjective and unreliable outcomes like pain, and existing compartmental pressure sensors are inaccurate and inefficient to use. MY01 measures pressure continuously whereas other compartmental pressure monitors are only equipped to provide single data points, requiring the patient to be stabbed with a needle whenever a new pressure measurement is required. This archaic method is extremely painful for the patient, and further complicates diagnosis of ACS. By providing timely monitoring of muscle pressure, MY01 helps doctors to reliably identify ACS and take action before irreversible complications occur.
Brusco: Under what circumstances would a patient require MY01?
Dr. Harvey: ACS occurs when muscle swelling occurs, usually post trauma. The pressure in the traumatized tissue exceeds perfusion pressure of the muscle. This cuts off blood supply to the affected muscles, usually following a high-energy trauma incident like a long-bone fracture. Such cases are frequently caused by sports injuries or motor-vehicle accidents though other major causes include crush injuries, falls, gunshot wounds, and burns. More recently, trauma centers have also noticed an increase in ACS attributable to long periods of unconsciousness and inactivity, which some research links to the opioid epidemic.
ACS is relatively rare but difficult to identify, and if affected muscles lack blood supply for too long, patients risk suffering permanent muscle damage or even limb amputation. Far too often, trauma patients tragically sustain long-term disabilities because of missed diagnoses. Not only does this impose a significant yet overlooked economic burden on the healthcare system, patients sustaining these unacceptable outcomes often suffer an unnecessary reduction in their post-injury quality of life.
Brusco: Why was this segment of the orthopedic trauma market deemed to be attractive?
Dr. Harvey:MY01 solves an unmet clinical need because doctors lack a simple, reliable way to screen trauma patients for ACS. The current standard for diagnosing ACS potentially causes so much harm to patients that it became extremely difficult to overlook. Our team is working to make MY01 the new standard for ACS care in trauma centers around the world.
In the United States alone, over 2.5 million people suffer long bone fractures every year. Of this patient population 500,000 injuries are reasonably susceptible to the development of ACS. We believe transforming ACS care from reactive emergencies into proactive interventions can add significant value for patients, surgeons, and hospitals.
Brusco: What are some future goals for MY01? (New indications, next-gen products, etc.)
Dr. Harvey:The MY01 mobile application will be updated continuously to improve workflows for clinicians and facilities. Users will be able to access a web portal where they can review patient data and upload their own notes and attachments, all in compliance with HIPAA, GDPR, and PIPEDA. Second, future target markets and indications for MY01 include abdominal compartment syndrome and intracranial compartment syndrome. Finally, a next-generation concept in development for MY01 is to supplement pressure-sensing with pH sensing, which could provide fundamental insights into the pathophysiology of ACS, in addition to addressing another major unmet clinical need which is the timely diagnosis of sepsis.
Brusco: Is there anything else you’d like to say regarding ACS diagnosis or the orthopedic trauma market?
Dr. Harvey:We’re excited about tackling a global challenge facing orthopedic trauma teams around the world. In accordance with our project scope, we’ve assembled a diverse team with a multidisciplinary skill set to execute on the delivery of a high performing device.
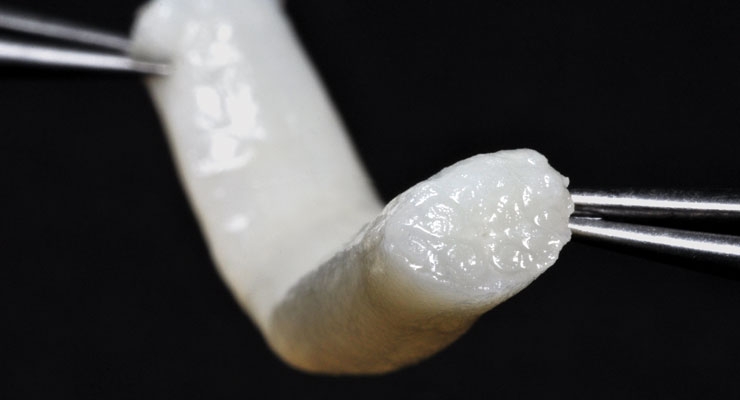
Restoring Lost Nerve Function
Sometimes a musculoskeletal injury affects more than just the muscle and bone. In some cases of traumatic injuries, a peripheral nerve (the motor and sensory nerves that connect the central nervous system to the entire body) may be injured, resulting in disrupted neurological signals and loss of normal body functions.
Unlike central nervous system injuries, treatment for peripheral nerve injury to restore function is possible. A less severely crushed or compressed nerve may heal on its own, but a seriously damaged or severed peripheral nerve will require surgical intervention to heal. Traditionally, a transected peripheral nerve would be repaired via a graft from another part of the body, but this can cause loss of function or sensation at that site.
To gain further understanding of the steps to repair these nerves, ODT spoke with Karen Zaderej, chairman, CEO, and president of AxoGen, an Alachua, Fla.-based company focused on the science, development, and commercialization of technologies for peripheral nerve regeneration and repair.
Brusco: Under what circumstances would a patient require peripheral nerve repair?
Zaderej: Every year, over 1.4 million people in the United States and millions more around the world experience loss of feeling, loss of movement, or chronic pain as a result of injuries to their peripheral nerves.1,2 There are many causes of nerve damage—from accidents that lead to traumatic injury, to nerve compression like carpal tunnel syndrome, to surgical procedures. Any of these can leave patients with a reduced quality of life.
Damaged peripheral nerves can be surgically repaired in a variety of ways, depending on the nature of the injury and the size of the nerve gap. We are solely dedicated to revolutionizing the science of peripheral nerve repair, giving surgeons new options to serve a patient population with significant unmet needs.
Brusco: How does AxoGen’s peripheral nerve repair technology work? What other technologies does AxoGen offer?
Zaderej:Our platform for peripheral nerve repair features a comprehensive portfolio of products designed to offer surgeons the opportunity to repair damaged nerves with fewer potential complications than the traditional surgical approach. The surgical techniques used may help prevent the onset of pain and other deficits that often result from nerve damage, while also reducing procedure costs.
Current standard-of-care procedures for long-gap nerve injuries often involve removing a nerve from another part of the patient’s body in order to repair the damaged nerve. This can cause a loss of function and sensation at the donor site. Avance Nerve Graft, our processed human nerve allograft, eliminates the need for a second surgical site and the comorbidities associated with harvesting a nerve during autograft procedures.
Repair of small-gap injuries frequently results in tension at the repair site—this can inhibit proper regeneration, resulting in impaired function and risk of pain. AxoGuard Nerve Connector enables what is called connector-assisted repair, tensionless repair for those small-gap nerve injuries.
Damaged nerves also require protection from soft tissue attachments and relief from inflammation to heal properly. AxoGuard Nerve Protector isolates and protects the injured nerve, minimizing the potential for soft tissue attachments and nerve entrapment, and Avive Soft Tissue Membrane helps modulate inflammation during the healing process.
Neurosensory evaluation and measurement tools are also essential to assist healthcare professionals in detecting changes in sensation, assessing return of sensation and function, evaluating effective treatments, and providing feedback to patients. AcroVal Neurosensory & Motor Testing System, a nerve function evaluation tool designed to be used by clinicians in the measurement, mapping, and monitoring of patients with peripheral nerve injuries, can be used for this purpose. AxoTouch Two-Point Discriminator can also be used for measuring sensation after a nerve injury, following the progression of a repaired nerve and in evaluation of possible nerve damage.
Brusco: Why was the peripheral nerve repair market deemed to be attractive?
Zaderej:We saw, and continue to see, a tremendous untapped opportunity to make a meaningful difference for millions of people who suffer the effects of peripheral nerve damage. Nerve repair in the extremities due to traumatic injury or nerve compression is currently our most developed clinical application. However, most of the company’s active accounts are at an early stage of penetration, which allows for continued market expansion and growth. There are more than 900,000 nerve repair surgeries annually in the United States, pointing to a market opportunity of over $2.2 billion for our products.3
Given the vast network of peripheral nerves in the body, the opportunity to apply our solutions to additional areas of nerve repair is substantial. Beyond extremity trauma and compression injuries, we are expanding adoption of our products in a wide variety of nerve repair surgeries, including oral and maxillofacial applications and our latest initiative, breast reconstruction neurotization for breast cancer survivors through a pioneering surgical method known as ReSensation.
Brusco: What is the future of peripheral nerve repair technology?
Zaderej: We will continue to work with leading researchers to further translate scientific data and clinical evidence into techniques and technologies that improve quality of life for patients with peripheral nerve damage. For example, the root cause of many cases of chronic pain can be traced to a damaged or entrapped nerve. So, we are actively exploring how our platform for nerve repair can best support clinicians and patients in the surgical management of pain.
Because expertise from a nerve specialist must be sought quickly to achieve optimal surgical outcomes in peripheral nerve repair, patient and clinician education is paramount to increase availability and adoption of modern nerve care. But with a strong commitment to developing clinical evidence and educating doctors about emerging best practices in the field, we have the necessary framework in place to change the paradigm of nerve repair to the benefit of patients and healthcare providers. We plan to continue investing in the scientific research, clinical studies, and physician education that have become the backbone of the company’s innovations.
Brusco: Is there anything else you’d like to say regarding peripheral nerve repair or the trauma market?
Zaderej:Our commitment to improved patient outcomes has been at the core of our business since the company’s founding and still fuels our work today. Currently, many patients and surgeons accept the loss of sensory and motor function as an inevitable outcome of traumatic injury. People who experience damage to their peripheral nerves are often unaware of the cause of their pain, numbness or loss of movement, and many clinicians who treat these patients are not aware of the impact of nerve damage on patients’ quality of life, or advances in technology that can repair nerve damage with fewer complications. As the company’s investment in awareness and education drives more clinicians to adopt our technologies and techniques for extremity nerve repair, more patients will have access to solutions focused on improving their quality of life.
References
It’s no surprise, then, that the trauma product market continues to thrive simply because three out of five people involved in a physical mishap will require some degree of orthopedic care. Some of those will need technology to assist in their recovery, fueling the trauma products market that B2B research firm MarketsandMarkets expects to grow 7.1 percent to $8.43 billion by the year 2022. This growth was attributed to a high prevalence of bone degenerative diseases, increasing incidence of road accidents and falls, and rising amounts of sports injuries.
Traditional technologies used to treat traumatic orthopedic injuries include internal fixators (plates, screws, nails, pins, wires, and staples), external fixators (unilateral and bilateral, circular, and hybrid), and a variety of other diverse trauma products. Orthopedic surgeons typically prefer internal fixators because they are used for fractures that can only be surgically repaired (i.e., unstable, pathological, and multiple fractures). External fixators are used in cases of open fractures, infected fractures, closed fractures, burns, and damage control orthopedics. Hospitals and trauma centers as well as ambulatory surgical centers (ASCs) are the primary end-users of these types of technologies.
The market is highly competitive, with both small and large players seeking a share. This article will explore three companies taking an innovative approach to developing technologies that assist patients when accidents happen.
Streamlining Efficiency and Value in the OR
Operating rooms are one of the costliest areas for most hospitals. Hospitals face a range of mounting financial pressures, so many are reexamining OR operations for avoidable costs. And as care and reimbursement moves closer to value-based models, orthopedic surgeons are continuing to seek out tools and resources to provide patients with the best care while lowering the cost of delivery.
Value-based care could perhaps have the largest effect on trauma and fracture patients. This type of care is emergent or urgent, and as a result, can have a significant number of unfunded or underfunded patients. However, the most expensive factor involved in fracture care surgery is typically the cost of the implant. In fact, many uninsured or underfunded patients are unable to have surgeries performed at outpatient centers because of prohibitive implant costs. With rising healthcare costs and decreasing physician and hospital reimbursement, this model isn’t sustainable in the long-term.
To gain more insight on this, ODT spoke with Steve Lichtenthal, vice president of business development for The Orthopaedic Implant Company (OIC), a Reno, Nev.-based orthopedic trauma implant manufacturer.
Brusco: What is unique about The Orthopaedic Implant Company’s orthopedic trauma portfolio?
Lichtenthal:Our orthopedic trauma portfolio was designed to bring efficiency and value in the OR. Oftentimes streamlined manufacturability and operating room efficiency is not considered a factor in orthopedic device design, resulting in the devices being far more expensive than their functional value would suggest. Our trauma portfolio is designed to be simple and straightforward. By and large, conventional vendors design implants and systems to validate the need for representation in the OR and hospital supply chain. Our engineering team is tasked with making things as intuitive and predictable as possible. This approach mitigates the need for vendor representation in the OR and in the hospital’s supply chain as well.
Brusco: OIC states that it is “…driven to change the way implants are manufactured and priced.” Can you elaborate on that? How does it apply to your trauma portfolio?
Lichtenthal: The measures that we have taken to create our unique model of delivery makes it possible to accomplish our primary goal—to make value-based care for patients and hospitals a reality. Our model halves implant spending. It is important to note that studies have proven our implants to be clinically equivalent to comparable, premium brand implants on the market today. Furthermore, these studies have documented and proven the value as well. The healthcare landscape is quickly moving from fee-for-service to value-based care models. We have committed to being part of the solution to help providers maintain standards in clinical outcomes, while helping them best the challenges value-based care presents.
Brusco: What are some future goals for the company’s trauma portfolio? (Next-gen products, new indications, etc.)
Lichtenthal: We have been very pleased with the adoption of our model. From rural hospitals to level 1 academic centers in urban areas, our value proposition has been proven to be a growing mainstay in the market. As our relationships with surgeons and facilities strengthen, we get incredible insight and feedback on how we should expand our offering. While we continue to add to our trauma portfolio, next year the market will see our first implant that goes beyond orthopedic trauma. Largely untouched by high-value vendors, this area of orthopedics is clamoring for savings and the impact is going to be incredible.
Brusco: Is there anything else you’d like to say regarding orthopedic trauma solutions or the orthopedic trauma market?
Lichtenthal: The market is quickly changing to reward value. That’s a first for orthopedic implants and the broader healthcare market as well. All of the vendors understand it, although few are willing to step up and take the reins. Most vendors’ strategies revolve around price preservation, betting on the rift between surgeons’ and hospitals’ goals and motivations. The reality is that rift is narrowing quickly and high-value implants are just an example of a bridge that leads to success for everyone—most importantly, the patient.
Transforming High-Energy Trauma Care
A condition called compartment syndrome can occur when muscle pressure builds to dangerous levels. This pressure can decrease blood flow, preventing oxygen from reaching nerve and muscle cells. It can either be acute or chronic, and acute compartment syndrome (ACS) is usually caused by a severe injury—a fracture, badly bruised muscle, or crush injury, for example. Left untreated, ACS can lead to permanent muscle damage. The problem is, diagnosing ACS relies on subjective pain measurements and often inaccurate sensing technology.
To further explore this, ODT spoke with Dr. Edward J. Harvey H.BSc, MDCM, MSc, FRCSC and co-founder of MY01, which created a digital medical device to diagnose ACS. Nxtsens Microsystems, which helped to develop MY01, specializes in sensor technology. Nxtsens is on a mission to empower healthcare professionals with the ability to preempt severe medical conditions, improving patient outcomes. Based in Montreal, Quebec, their team specializes in next-generation cloud-enabled MEMS technology.
Brusco: How does MY01 work? How does it differ from other solutions to diagnose ACS?
Dr. Harvey: MY01 is a digitally connected medical device that aids in the diagnosis of acute compartment syndrome (ACS). Its insertion mechanism allows easy, accurate placement of a small pressure sensor inside a patient's muscle compartment. The sensor connects to a body-worn display screen through a biocompatible lead-wire, and continuously outputs accurate pressure values while resting on a patient’s skin. MY01 is accompanied by a mobile application that wirelessly connects to the medical device via Bluetooth. Our app transfers pressure data to hospital cloud systems, enabling remote data display, and also allows clinicians to log patient pain scores, notes, and images.
Currently, ACS is diagnosed based on subjective and unreliable outcomes like pain, and existing compartmental pressure sensors are inaccurate and inefficient to use. MY01 measures pressure continuously whereas other compartmental pressure monitors are only equipped to provide single data points, requiring the patient to be stabbed with a needle whenever a new pressure measurement is required. This archaic method is extremely painful for the patient, and further complicates diagnosis of ACS. By providing timely monitoring of muscle pressure, MY01 helps doctors to reliably identify ACS and take action before irreversible complications occur.
Brusco: Under what circumstances would a patient require MY01?
Dr. Harvey: ACS occurs when muscle swelling occurs, usually post trauma. The pressure in the traumatized tissue exceeds perfusion pressure of the muscle. This cuts off blood supply to the affected muscles, usually following a high-energy trauma incident like a long-bone fracture. Such cases are frequently caused by sports injuries or motor-vehicle accidents though other major causes include crush injuries, falls, gunshot wounds, and burns. More recently, trauma centers have also noticed an increase in ACS attributable to long periods of unconsciousness and inactivity, which some research links to the opioid epidemic.
ACS is relatively rare but difficult to identify, and if affected muscles lack blood supply for too long, patients risk suffering permanent muscle damage or even limb amputation. Far too often, trauma patients tragically sustain long-term disabilities because of missed diagnoses. Not only does this impose a significant yet overlooked economic burden on the healthcare system, patients sustaining these unacceptable outcomes often suffer an unnecessary reduction in their post-injury quality of life.
Brusco: Why was this segment of the orthopedic trauma market deemed to be attractive?
Dr. Harvey:MY01 solves an unmet clinical need because doctors lack a simple, reliable way to screen trauma patients for ACS. The current standard for diagnosing ACS potentially causes so much harm to patients that it became extremely difficult to overlook. Our team is working to make MY01 the new standard for ACS care in trauma centers around the world.
In the United States alone, over 2.5 million people suffer long bone fractures every year. Of this patient population 500,000 injuries are reasonably susceptible to the development of ACS. We believe transforming ACS care from reactive emergencies into proactive interventions can add significant value for patients, surgeons, and hospitals.
Brusco: What are some future goals for MY01? (New indications, next-gen products, etc.)
Dr. Harvey:The MY01 mobile application will be updated continuously to improve workflows for clinicians and facilities. Users will be able to access a web portal where they can review patient data and upload their own notes and attachments, all in compliance with HIPAA, GDPR, and PIPEDA. Second, future target markets and indications for MY01 include abdominal compartment syndrome and intracranial compartment syndrome. Finally, a next-generation concept in development for MY01 is to supplement pressure-sensing with pH sensing, which could provide fundamental insights into the pathophysiology of ACS, in addition to addressing another major unmet clinical need which is the timely diagnosis of sepsis.
Brusco: Is there anything else you’d like to say regarding ACS diagnosis or the orthopedic trauma market?
Dr. Harvey:We’re excited about tackling a global challenge facing orthopedic trauma teams around the world. In accordance with our project scope, we’ve assembled a diverse team with a multidisciplinary skill set to execute on the delivery of a high performing device.
Restoring Lost Nerve Function
Sometimes a musculoskeletal injury affects more than just the muscle and bone. In some cases of traumatic injuries, a peripheral nerve (the motor and sensory nerves that connect the central nervous system to the entire body) may be injured, resulting in disrupted neurological signals and loss of normal body functions.
Unlike central nervous system injuries, treatment for peripheral nerve injury to restore function is possible. A less severely crushed or compressed nerve may heal on its own, but a seriously damaged or severed peripheral nerve will require surgical intervention to heal. Traditionally, a transected peripheral nerve would be repaired via a graft from another part of the body, but this can cause loss of function or sensation at that site.
To gain further understanding of the steps to repair these nerves, ODT spoke with Karen Zaderej, chairman, CEO, and president of AxoGen, an Alachua, Fla.-based company focused on the science, development, and commercialization of technologies for peripheral nerve regeneration and repair.
Brusco: Under what circumstances would a patient require peripheral nerve repair?
Zaderej: Every year, over 1.4 million people in the United States and millions more around the world experience loss of feeling, loss of movement, or chronic pain as a result of injuries to their peripheral nerves.1,2 There are many causes of nerve damage—from accidents that lead to traumatic injury, to nerve compression like carpal tunnel syndrome, to surgical procedures. Any of these can leave patients with a reduced quality of life.
Damaged peripheral nerves can be surgically repaired in a variety of ways, depending on the nature of the injury and the size of the nerve gap. We are solely dedicated to revolutionizing the science of peripheral nerve repair, giving surgeons new options to serve a patient population with significant unmet needs.
Brusco: How does AxoGen’s peripheral nerve repair technology work? What other technologies does AxoGen offer?
Zaderej:Our platform for peripheral nerve repair features a comprehensive portfolio of products designed to offer surgeons the opportunity to repair damaged nerves with fewer potential complications than the traditional surgical approach. The surgical techniques used may help prevent the onset of pain and other deficits that often result from nerve damage, while also reducing procedure costs.
Current standard-of-care procedures for long-gap nerve injuries often involve removing a nerve from another part of the patient’s body in order to repair the damaged nerve. This can cause a loss of function and sensation at the donor site. Avance Nerve Graft, our processed human nerve allograft, eliminates the need for a second surgical site and the comorbidities associated with harvesting a nerve during autograft procedures.
Repair of small-gap injuries frequently results in tension at the repair site—this can inhibit proper regeneration, resulting in impaired function and risk of pain. AxoGuard Nerve Connector enables what is called connector-assisted repair, tensionless repair for those small-gap nerve injuries.
Damaged nerves also require protection from soft tissue attachments and relief from inflammation to heal properly. AxoGuard Nerve Protector isolates and protects the injured nerve, minimizing the potential for soft tissue attachments and nerve entrapment, and Avive Soft Tissue Membrane helps modulate inflammation during the healing process.
Neurosensory evaluation and measurement tools are also essential to assist healthcare professionals in detecting changes in sensation, assessing return of sensation and function, evaluating effective treatments, and providing feedback to patients. AcroVal Neurosensory & Motor Testing System, a nerve function evaluation tool designed to be used by clinicians in the measurement, mapping, and monitoring of patients with peripheral nerve injuries, can be used for this purpose. AxoTouch Two-Point Discriminator can also be used for measuring sensation after a nerve injury, following the progression of a repaired nerve and in evaluation of possible nerve damage.
Brusco: Why was the peripheral nerve repair market deemed to be attractive?
Zaderej:We saw, and continue to see, a tremendous untapped opportunity to make a meaningful difference for millions of people who suffer the effects of peripheral nerve damage. Nerve repair in the extremities due to traumatic injury or nerve compression is currently our most developed clinical application. However, most of the company’s active accounts are at an early stage of penetration, which allows for continued market expansion and growth. There are more than 900,000 nerve repair surgeries annually in the United States, pointing to a market opportunity of over $2.2 billion for our products.3
Given the vast network of peripheral nerves in the body, the opportunity to apply our solutions to additional areas of nerve repair is substantial. Beyond extremity trauma and compression injuries, we are expanding adoption of our products in a wide variety of nerve repair surgeries, including oral and maxillofacial applications and our latest initiative, breast reconstruction neurotization for breast cancer survivors through a pioneering surgical method known as ReSensation.
Brusco: What is the future of peripheral nerve repair technology?
Zaderej: We will continue to work with leading researchers to further translate scientific data and clinical evidence into techniques and technologies that improve quality of life for patients with peripheral nerve damage. For example, the root cause of many cases of chronic pain can be traced to a damaged or entrapped nerve. So, we are actively exploring how our platform for nerve repair can best support clinicians and patients in the surgical management of pain.
Because expertise from a nerve specialist must be sought quickly to achieve optimal surgical outcomes in peripheral nerve repair, patient and clinician education is paramount to increase availability and adoption of modern nerve care. But with a strong commitment to developing clinical evidence and educating doctors about emerging best practices in the field, we have the necessary framework in place to change the paradigm of nerve repair to the benefit of patients and healthcare providers. We plan to continue investing in the scientific research, clinical studies, and physician education that have become the backbone of the company’s innovations.
Brusco: Is there anything else you’d like to say regarding peripheral nerve repair or the trauma market?
Zaderej:Our commitment to improved patient outcomes has been at the core of our business since the company’s founding and still fuels our work today. Currently, many patients and surgeons accept the loss of sensory and motor function as an inevitable outcome of traumatic injury. People who experience damage to their peripheral nerves are often unaware of the cause of their pain, numbness or loss of movement, and many clinicians who treat these patients are not aware of the impact of nerve damage on patients’ quality of life, or advances in technology that can repair nerve damage with fewer complications. As the company’s investment in awareness and education drives more clinicians to adopt our technologies and techniques for extremity nerve repair, more patients will have access to solutions focused on improving their quality of life.
References
- “Health”, United States, 2011, Publication of US Department of Health & Human Services.
- Noble, et al., J of Trauma Injury Infection and Critical Care, 1998.
- Brattain K. (2013). Analysis of the Peripheral Nerve Repair Market in the United States