Vicki A. Barbur, Ph.D. and Anthony Duong, Ph.D., Battelle 08.14.19
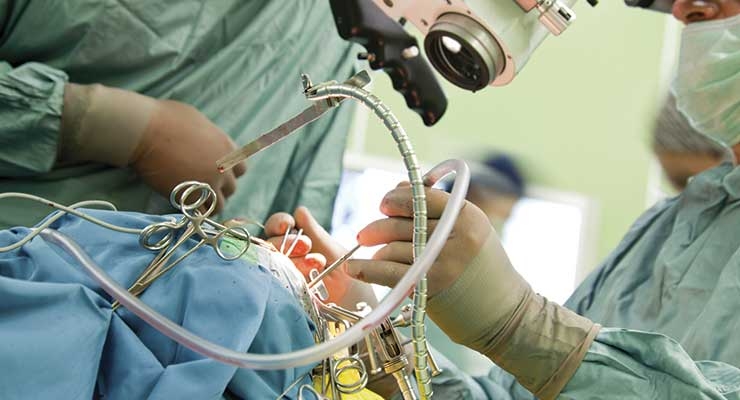
Each year, nearly 200,000 people in the U.S. undergo revision surgeries for hip and knee replacements. A significant fraction of these are due to complications from prosthetic joint infections (PJI).
Julio Zelaya, a researcher in the Advanced Materials group at Battelle, knew there had to be a better way to prevent PJI and reduce the need for revision surgeries. He became interested in the problem when his grandmother, Consuela, had her hip replaced in the summer of 2017. “It’s a hard process, especially for people who are elderly,” he noted. “Her recovery took months.”
To tackle the problem, Zalaya developed a proposal to research ortho-coatings for prosthetic joints that could deliver sustained, localized doses of antimicrobials to stop PJI in its tracks. His pitch won first place at Battelle’s annual Innovation Gathering, where proposals for internal research and development projects are evaluated.
Now, this vision is becoming a reality. A team at Battelle, led by Zelaya and under the supervision of Principal Research Scientist Anthony Duong, has developed a novel ortho-coating that could reduce the incidence of PJI and improve the odds of surgical implant success. The coating provides sustained release of antimicrobials to prevent and treat joint infections right at the source, before they require expensive and painful intervention.
Prosthetic Joint Infections: Risks, Costs, and Treatments
Total joint replacement is one of the most commonly performed elective surgeries in the U.S., with more than 1 million patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) each year.1 About 12 percent of them will require revision surgery within 10 years, with most revisions performed in the first 18 months after the original surgery.2
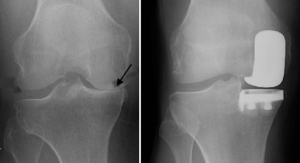
Some of these revisions are required to address problems that arise due to mechanical failures in the artificial joint, damage caused by falls or other injuries, or wear and tear on an older joint. A significant percentage of revision surgeries, however, are required to treat infections that colonize the surface of the joint. Roughly 2 percent of TKA or THA surgeries result in PJI.3 For revision surgeries, the rate is even higher, with more than 6 percent of patients experiencing infections after a surgical revision.4 Infections can arise at any time, yet are most common in the first six weeks following surgery. Post-surgical infections arise when bacteria colonize the surface of the new implant.
When a prosthetic joint is implanted, the body responds by coating the surface with serum proteins that support cell growth and tissue repair. In the normal course of healing, this surface is colonized by osteoblasts—specialized cells that secrete a matrix for bone formation. Over time, the body builds new bone and cartilage that forms a direct interface between the implant and the bone, a process known as osseointegration. This integration is essential for the long-term functioning of the joint; the new tissue prevents rejection of the artificial joint, increases strength and stability, and reduces the risk of aseptic loosening of the joint. Artificial joints are made of biocompatible materials and typically have specialized surface treatments or coatings to support the osseointegration process.
Unfortunately, the same conditions that support new tissue formation are also attractive to pathogens. This results in a “race to the surface” as osteoblasts and bacteria compete to colonize the surface of the joint. If microbes outcompete the osteoblasts, a joint infection will take hold. As bacteria multiply, they can form a biofilm on the surface of the joint that is difficult to penetrate and disperse with antibiotics.
Once the infection reaches this point, surgery is often required to clear the infection. If caught in the early stages, the joint can sometimes be left in place and the infection cleared by aggressive debridement (removing infected tissue and thoroughly cleaning by rinsing) followed by an intensive course of oral or intravenous antibiotics—a treatment protocol known as debridement, antibiotics, and implant retention (DAIR). However, this treatment has mixed results, with some studies showing success rates as low as 33 to 52 percent.3
For infections that cannot be controlled through DAIR, or in cases where DAIR has not resulted in eradication of the infection, patients may undergo a revision surgery in which the infected joint is removed, and a new joint is implanted. The most common protocol in the U.S. is a two-stage arthroplasty exchange, or staged exchange. In the first surgery, the infected joint is removed, the area is aggressively debrided, and a spacer impregnated with antimicrobials is implanted in place of the joint. The spacer stabilizes the joint and enhances patient comfort while delivering localized antibiotic treatment. The new joint is implanted four to six weeks later, following intensive treatment with intravenous antibiotics. Oral antibiotics may be used for an extended period after the surgery.
Two-stage arthroplasty exchange has a much higher success rate than DAIR, but it is costly, highly uncomfortable, and disruptive for the patient. Patients may have to be kept largely immobile for six weeks or longer during the two-stage process. Extended courses of broad-spectrum, systemic antimicrobials have negative effects on the natural microbiome that can have long-term consequences for patients, in addition to contributing to the rise of antibiotic-resistant strains of bacteria.
Revision surgeries are cost intensive and, because of Medicare and Medicaid reimbursement formulas, are often performed at a loss for the hospital. In 2009, the direct costs of PJI treatments to the U.S. healthcare system were estimated to be $566 million.5 These costs have undoubtedly risen significantly over the last 10 years with inflation and the increasing number of patients seeking joint replacement surgery.
The economic and non-economic costs associated with PJI make finding preventative strategies a priority. The most promising strategies attack PJI at the source by preventing the buildup of microbes and biofilms on the prosthetic joint.
Reviewing Existing Ortho-Coatings
Surgical implants for hip and knee replacement must be designed to reduce medical complications and hold up under years of potentially strenuous use. Therefore, the chosen materials must have specialized characteristics. Specifically, they must be:
A Sustained-Release Antimicrobial Ortho-Coating
A tunable, extended-release coating would provide significant benefits for patients, healthcare providers, and medical device manufacturers. PJI doesn’t always develop immediately after surgery. Infection often builds up over time, taking weeks or months to develop. These infections can incubate in the joint and surrounding tissue for a long time before symptoms—usually in the form of pain and/or aseptic loosening—become obvious to patients and care providers. By this point, it is often too late to control the infection with non-surgical antibiotic therapies.
A sustained release coating could reduce the risk of PJI in the weeks and months following the surgical implant.
Battelle’s coating consists of a bioinert polymer with a micro-featured surface. This formulation combines antimicrobial and osseointegration-promoting properties. The surface features stimulate adhesion and integration of osteoblasts for improved bone ingrowth at the implant interface. The structures are produced through micro-embossing or electrospinning techniques. Growth factors are immobilized on the surface as actives to induce production of new bone material. Additionally, the polymer releases active ingredients slowly over time through biodegradation or diffusion. The active ingredients can be tuned for targeted, sustained local delivery of specified antimicrobials to the implant surface. The coating also incorporates immune-modulating actives to reduce scarring and improve natural immune response at the interface.
The coating builds on Battelle’s previous work in advanced materials (especially surfaces and interfaces), medical device development, and drug delivery. In particular, the controlled release polymer has its roots in another Battelle project: Acute Care Cover for Severely Injured Limbs (ACCSIL). ACCSIL, created for the U.S. military, was developed to reduce the risk of infections that develop after traumatic limb injuries in battlefield conditions, which often result in amputations. Soldiers may need to be stabilized in the field for up to 72 hours before they can be evacuated to a medical facility. ACCSIL stops bleeding, prevents infection, and delivers oxygen to the wound to prevent tissue death. An oxygen-generating hydrogel is triggered when the dressing is applied to deliver a controlled release of oxygen over a 72-hour period.
The polymer used in the ortho-coating uses similar technology to deliver antimicrobials over an extended period. It starts acting immediately and continues to deliver controlled doses of the antimicrobial over the next several months. This gets patients through the most dangerous post-surgical period when most infections develop. The polymer can be tuned to deliver a variety of active compounds, including different antimicrobials, immune-modulators, and cell signaling factors.
Building a Better Ortho-Coating
The coating consists of a polymer with surface morphology features on the micro- and nano-scales. The polymer may be any in a range of thermosetting or curing polymers. The surface morphology is created either by embossing the pattern into a biopolymer via a mold technique or through an electrospinning technique, in which a biopolymer is spun into fibers through an electrohydrodynamic jet and precipitated onto the surface of a grounded target implant. These fibers mimic the microenvironment that favors osteoblast adhesion; a wide variety of surface structures can be generated to improve osseointegration and new bone formation. Bioresorbable polymers can be created, which act as a scaffold for autologous cells and then biodegrade once enough bone has regenerated.
The polymers can be designed to release active ingredients in one of two ways. One approach leads to sustained release through a swelling diffusion mechanism from the polymer surface itself. Under these conditions, the inert biopolymer would be silicone-based and designed to absorb water from the body. As the water diffuses into the silicon, the antimicrobial active diffuses out through the water-filled matrix. An alternative approach relies on a polymer with bioresorbable characteristics that hydrolyzes and dissolves within the body over time. As the polymer degrades, the antimicrobial is released. The rate of release can be tuned by the polymer chemistry, molecular weight, and blend.
Polymers can be designed to release antimicrobial active agents including (but not limited to) gentamicin, neomycin, and vancomycin. These agents can be embedded directly into the system. In addition to antimicrobials, the coatings could also be loaded with immune-modulating actives, which could include immune-stimulants such as imiquimod and resiquimod, or immune-suppressants such as dexamethasone. These modulators would prevent biofouling and scarring, and improve immune cell signaling. Coatings could potentially even be loaded with cell-signaling cytokines and growth factors, such as platelet-rich-plasma-derived growth factors. These actives signal osteogenic cells to differentiate and/or produce bone material once they are integrated into the surface morphology of the coating. These growth factors can be immobilized on the surface of the implant via a layer-by-layer deposition technique to optimize conditions for bone growth.
The combined features of the coating provide several potential benefits that could significantly improve outcomes for arthroplasty surgeries.
The micro- and nano-featured surface, combined with growth factors immobilized on the surface, provides better conditions for osseointegration and bone formation than conventional HA coatings.
Controlled, sustained, and local release of antibiotics over the critical first months after surgery could reduce rates of post-surgery PJI, which would result in faster recovery times and fewer infection-related revision surgeries.
Local delivery of antibiotics has a “dose sparing” effect, meaning lower doses of antibiotics can be used. This reduces concerns about antibiotic overuse, including off-target effects associated with using systemic antibiotics over extended time periods and the risk of developing antibiotic-resistant strains of bacteria.
Immune-modulating actives prevent the buildup of excessive scar tissue around the implant and signal necessary immune cells to support healthy immune response.
Better Outcomes for Total Joint Replacement Patients
Improved ortho-coatings that can deliver controlled doses of antimicrobials and other actives over time would have tremendous benefits for patients, hospitals, and device manufacturers. Reducing the incidence of PJI would also reduce the need for infection-related revision surgeries. Where revision surgery is needed to replace worn or damaged prosthetics, the use of controlled-release antimicrobial coatings could potentially allow surgeons to move from two-stage arthroplasty exchange to a one-stage process that eliminates the spacer step and second surgery. This would get patients back on their feet much faster, reduce pain and disruption to their lives, and significantly reduce the costs associated with revision surgeries.
Hospitals are currently under tremendous pressure from insurers and the Centers for Medicare and Medicaid Services (CMS) to improve patient outcomes, reduce rates of re-hospitalization, and demonstrate the value and efficacy of the treatments they provide. An ortho-coating that can help hospitals achieve these outcomes would have a significant competitive advantage, in addition to being better for patients.
The technology is still in its infancy, and Battelle is seeking collaborators to move it forward through the next stages of development, testing, and FDA approval.
Bringing the coating to market would be a dream come true for Zelaya. “It’s about reducing pain and anxiety and improving outcomes for real people,” he said—real people like his grandmother, and the hundreds of thousands of patients like her who undergo total joint replacement surgeries each year.
Note: Companies interested in exploring licensing or co-development of this novel, new coating material can contact Vicki Barbur at barbur@battelle.org.
References
Vicki A. Barbur, Ph.D. is the senior director of IP and technology commercialization at Battelle. She brings dual expertise in science and business as well as broad experience in several technical disciplines to her role associated with technology commercialization and IP management. Her primary areas of focus are health and medical devices, genomics and biosecurity, as well as energy and environment. Barbur’s work also includes streamlining the process for securing intellectual property and licensing to allow external organizations and companies to put innovation to use quickly as well as developing collaborative and strategic partnerships. Previously, Barbur was SVP and CTO for Concurrent Technologies Corporation and VP, R&D for Cardinal Health. Barbur earned a Ph.D. and BSc in physics from Imperial College, University of London, and an MSc in applied statistics from the University of Oxford, both in the U.K.
Anthony Duong, Ph.D. is a principal research scientist for materials science at Battelle. He has diverse expertise spanning coatings, nanotechnology, encapsulation, controlled release, and aerosols. Currently, Duong is developing concepts and technology for encapsulation and controlled release and is particularly interested in leveraging drug delivery technology from the pharmaceutical industry to meet consumer market needs in encapsulation and controlled release. Before joining Battelle in December 2013, Duong received his Ph.D. from Ohio State in chemical engineering. Subsequently, he carried out post-doctoral research in the Ohio State College of Pharmacy investigating drug delivery for immunology applications, including the development of vaccines and sustained release antimicrobials. Duong’s dissertation focused on processing and production of nanoparticles for drug delivery, imaging, and in-vitro cell detection and manipulation. He has developed novel processes for encapsulating bioactive agents in pH sensitive biocompatible polymer microparticles and liposomes.
Julio Zelaya, a researcher in the Advanced Materials group at Battelle, knew there had to be a better way to prevent PJI and reduce the need for revision surgeries. He became interested in the problem when his grandmother, Consuela, had her hip replaced in the summer of 2017. “It’s a hard process, especially for people who are elderly,” he noted. “Her recovery took months.”
To tackle the problem, Zalaya developed a proposal to research ortho-coatings for prosthetic joints that could deliver sustained, localized doses of antimicrobials to stop PJI in its tracks. His pitch won first place at Battelle’s annual Innovation Gathering, where proposals for internal research and development projects are evaluated.
Now, this vision is becoming a reality. A team at Battelle, led by Zelaya and under the supervision of Principal Research Scientist Anthony Duong, has developed a novel ortho-coating that could reduce the incidence of PJI and improve the odds of surgical implant success. The coating provides sustained release of antimicrobials to prevent and treat joint infections right at the source, before they require expensive and painful intervention.
Prosthetic Joint Infections: Risks, Costs, and Treatments
Total joint replacement is one of the most commonly performed elective surgeries in the U.S., with more than 1 million patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) each year.1 About 12 percent of them will require revision surgery within 10 years, with most revisions performed in the first 18 months after the original surgery.2
Some of these revisions are required to address problems that arise due to mechanical failures in the artificial joint, damage caused by falls or other injuries, or wear and tear on an older joint. A significant percentage of revision surgeries, however, are required to treat infections that colonize the surface of the joint. Roughly 2 percent of TKA or THA surgeries result in PJI.3 For revision surgeries, the rate is even higher, with more than 6 percent of patients experiencing infections after a surgical revision.4 Infections can arise at any time, yet are most common in the first six weeks following surgery. Post-surgical infections arise when bacteria colonize the surface of the new implant.
When a prosthetic joint is implanted, the body responds by coating the surface with serum proteins that support cell growth and tissue repair. In the normal course of healing, this surface is colonized by osteoblasts—specialized cells that secrete a matrix for bone formation. Over time, the body builds new bone and cartilage that forms a direct interface between the implant and the bone, a process known as osseointegration. This integration is essential for the long-term functioning of the joint; the new tissue prevents rejection of the artificial joint, increases strength and stability, and reduces the risk of aseptic loosening of the joint. Artificial joints are made of biocompatible materials and typically have specialized surface treatments or coatings to support the osseointegration process.
Unfortunately, the same conditions that support new tissue formation are also attractive to pathogens. This results in a “race to the surface” as osteoblasts and bacteria compete to colonize the surface of the joint. If microbes outcompete the osteoblasts, a joint infection will take hold. As bacteria multiply, they can form a biofilm on the surface of the joint that is difficult to penetrate and disperse with antibiotics.
Once the infection reaches this point, surgery is often required to clear the infection. If caught in the early stages, the joint can sometimes be left in place and the infection cleared by aggressive debridement (removing infected tissue and thoroughly cleaning by rinsing) followed by an intensive course of oral or intravenous antibiotics—a treatment protocol known as debridement, antibiotics, and implant retention (DAIR). However, this treatment has mixed results, with some studies showing success rates as low as 33 to 52 percent.3
For infections that cannot be controlled through DAIR, or in cases where DAIR has not resulted in eradication of the infection, patients may undergo a revision surgery in which the infected joint is removed, and a new joint is implanted. The most common protocol in the U.S. is a two-stage arthroplasty exchange, or staged exchange. In the first surgery, the infected joint is removed, the area is aggressively debrided, and a spacer impregnated with antimicrobials is implanted in place of the joint. The spacer stabilizes the joint and enhances patient comfort while delivering localized antibiotic treatment. The new joint is implanted four to six weeks later, following intensive treatment with intravenous antibiotics. Oral antibiotics may be used for an extended period after the surgery.
Two-stage arthroplasty exchange has a much higher success rate than DAIR, but it is costly, highly uncomfortable, and disruptive for the patient. Patients may have to be kept largely immobile for six weeks or longer during the two-stage process. Extended courses of broad-spectrum, systemic antimicrobials have negative effects on the natural microbiome that can have long-term consequences for patients, in addition to contributing to the rise of antibiotic-resistant strains of bacteria.
Revision surgeries are cost intensive and, because of Medicare and Medicaid reimbursement formulas, are often performed at a loss for the hospital. In 2009, the direct costs of PJI treatments to the U.S. healthcare system were estimated to be $566 million.5 These costs have undoubtedly risen significantly over the last 10 years with inflation and the increasing number of patients seeking joint replacement surgery.
The economic and non-economic costs associated with PJI make finding preventative strategies a priority. The most promising strategies attack PJI at the source by preventing the buildup of microbes and biofilms on the prosthetic joint.
Reviewing Existing Ortho-Coatings
Surgical implants for hip and knee replacement must be designed to reduce medical complications and hold up under years of potentially strenuous use. Therefore, the chosen materials must have specialized characteristics. Specifically, they must be:
- Durable and wear-resistant, so the part will not break down in the body or produce derivatives that could have adverse effects on patients.
- Low friction, so the joint operates smoothly and comfortably for the patient.
- Biocompatible and chemically inert to minimize chances of rejection or adverse reactions by the patient’s immune system.
- Bioceramics and bioactive coatings that promote bone ingrowth/osseointegration and improve biocompatibility.
- Coatings infused with silver or other materials with anti-microbial properties to reduce bacterial adhesion.
- Coatings that release antimicrobial medications.
- Sintered-bead coatings, which are created by applying a powder to the surface of the implant and heating it to just below the melting temperature to form a bond. Sintered-bead coatings create a rough, porous surface that promotes better bone ingrowth by providing more surfaces for osteoblasts to adhere to.
- Plasma-sprayed hydroxyapatite (HA), which is a phase of calcium phosphate—an inorganic material found naturally in bones. This coating can be sprayed onto a variety of base materials to improve biocompatibility and osseointegration. HA coatings can connect structurally and functionally to human bone.
A Sustained-Release Antimicrobial Ortho-Coating
A tunable, extended-release coating would provide significant benefits for patients, healthcare providers, and medical device manufacturers. PJI doesn’t always develop immediately after surgery. Infection often builds up over time, taking weeks or months to develop. These infections can incubate in the joint and surrounding tissue for a long time before symptoms—usually in the form of pain and/or aseptic loosening—become obvious to patients and care providers. By this point, it is often too late to control the infection with non-surgical antibiotic therapies.
A sustained release coating could reduce the risk of PJI in the weeks and months following the surgical implant.
Battelle’s coating consists of a bioinert polymer with a micro-featured surface. This formulation combines antimicrobial and osseointegration-promoting properties. The surface features stimulate adhesion and integration of osteoblasts for improved bone ingrowth at the implant interface. The structures are produced through micro-embossing or electrospinning techniques. Growth factors are immobilized on the surface as actives to induce production of new bone material. Additionally, the polymer releases active ingredients slowly over time through biodegradation or diffusion. The active ingredients can be tuned for targeted, sustained local delivery of specified antimicrobials to the implant surface. The coating also incorporates immune-modulating actives to reduce scarring and improve natural immune response at the interface.
The coating builds on Battelle’s previous work in advanced materials (especially surfaces and interfaces), medical device development, and drug delivery. In particular, the controlled release polymer has its roots in another Battelle project: Acute Care Cover for Severely Injured Limbs (ACCSIL). ACCSIL, created for the U.S. military, was developed to reduce the risk of infections that develop after traumatic limb injuries in battlefield conditions, which often result in amputations. Soldiers may need to be stabilized in the field for up to 72 hours before they can be evacuated to a medical facility. ACCSIL stops bleeding, prevents infection, and delivers oxygen to the wound to prevent tissue death. An oxygen-generating hydrogel is triggered when the dressing is applied to deliver a controlled release of oxygen over a 72-hour period.
The polymer used in the ortho-coating uses similar technology to deliver antimicrobials over an extended period. It starts acting immediately and continues to deliver controlled doses of the antimicrobial over the next several months. This gets patients through the most dangerous post-surgical period when most infections develop. The polymer can be tuned to deliver a variety of active compounds, including different antimicrobials, immune-modulators, and cell signaling factors.
Building a Better Ortho-Coating
The coating consists of a polymer with surface morphology features on the micro- and nano-scales. The polymer may be any in a range of thermosetting or curing polymers. The surface morphology is created either by embossing the pattern into a biopolymer via a mold technique or through an electrospinning technique, in which a biopolymer is spun into fibers through an electrohydrodynamic jet and precipitated onto the surface of a grounded target implant. These fibers mimic the microenvironment that favors osteoblast adhesion; a wide variety of surface structures can be generated to improve osseointegration and new bone formation. Bioresorbable polymers can be created, which act as a scaffold for autologous cells and then biodegrade once enough bone has regenerated.
The polymers can be designed to release active ingredients in one of two ways. One approach leads to sustained release through a swelling diffusion mechanism from the polymer surface itself. Under these conditions, the inert biopolymer would be silicone-based and designed to absorb water from the body. As the water diffuses into the silicon, the antimicrobial active diffuses out through the water-filled matrix. An alternative approach relies on a polymer with bioresorbable characteristics that hydrolyzes and dissolves within the body over time. As the polymer degrades, the antimicrobial is released. The rate of release can be tuned by the polymer chemistry, molecular weight, and blend.
Polymers can be designed to release antimicrobial active agents including (but not limited to) gentamicin, neomycin, and vancomycin. These agents can be embedded directly into the system. In addition to antimicrobials, the coatings could also be loaded with immune-modulating actives, which could include immune-stimulants such as imiquimod and resiquimod, or immune-suppressants such as dexamethasone. These modulators would prevent biofouling and scarring, and improve immune cell signaling. Coatings could potentially even be loaded with cell-signaling cytokines and growth factors, such as platelet-rich-plasma-derived growth factors. These actives signal osteogenic cells to differentiate and/or produce bone material once they are integrated into the surface morphology of the coating. These growth factors can be immobilized on the surface of the implant via a layer-by-layer deposition technique to optimize conditions for bone growth.
The combined features of the coating provide several potential benefits that could significantly improve outcomes for arthroplasty surgeries.
The micro- and nano-featured surface, combined with growth factors immobilized on the surface, provides better conditions for osseointegration and bone formation than conventional HA coatings.
Controlled, sustained, and local release of antibiotics over the critical first months after surgery could reduce rates of post-surgery PJI, which would result in faster recovery times and fewer infection-related revision surgeries.
Local delivery of antibiotics has a “dose sparing” effect, meaning lower doses of antibiotics can be used. This reduces concerns about antibiotic overuse, including off-target effects associated with using systemic antibiotics over extended time periods and the risk of developing antibiotic-resistant strains of bacteria.
Immune-modulating actives prevent the buildup of excessive scar tissue around the implant and signal necessary immune cells to support healthy immune response.
Better Outcomes for Total Joint Replacement Patients
Improved ortho-coatings that can deliver controlled doses of antimicrobials and other actives over time would have tremendous benefits for patients, hospitals, and device manufacturers. Reducing the incidence of PJI would also reduce the need for infection-related revision surgeries. Where revision surgery is needed to replace worn or damaged prosthetics, the use of controlled-release antimicrobial coatings could potentially allow surgeons to move from two-stage arthroplasty exchange to a one-stage process that eliminates the spacer step and second surgery. This would get patients back on their feet much faster, reduce pain and disruption to their lives, and significantly reduce the costs associated with revision surgeries.
Hospitals are currently under tremendous pressure from insurers and the Centers for Medicare and Medicaid Services (CMS) to improve patient outcomes, reduce rates of re-hospitalization, and demonstrate the value and efficacy of the treatments they provide. An ortho-coating that can help hospitals achieve these outcomes would have a significant competitive advantage, in addition to being better for patients.
The technology is still in its infancy, and Battelle is seeking collaborators to move it forward through the next stages of development, testing, and FDA approval.
Bringing the coating to market would be a dream come true for Zelaya. “It’s about reducing pain and anxiety and improving outcomes for real people,” he said—real people like his grandmother, and the hundreds of thousands of patients like her who undergo total joint replacement surgeries each year.
Note: Companies interested in exploring licensing or co-development of this novel, new coating material can contact Vicki Barbur at barbur@battelle.org.
References
- http://bit.ly/odt190721
- http://bit.ly/odt190722
- http://bit.ly/odt190723
- http://bit.ly/odt190724
- http://bit.ly/odt190725
- http://bit.ly/odt190726
- http://bit.ly/odt190727
Vicki A. Barbur, Ph.D. is the senior director of IP and technology commercialization at Battelle. She brings dual expertise in science and business as well as broad experience in several technical disciplines to her role associated with technology commercialization and IP management. Her primary areas of focus are health and medical devices, genomics and biosecurity, as well as energy and environment. Barbur’s work also includes streamlining the process for securing intellectual property and licensing to allow external organizations and companies to put innovation to use quickly as well as developing collaborative and strategic partnerships. Previously, Barbur was SVP and CTO for Concurrent Technologies Corporation and VP, R&D for Cardinal Health. Barbur earned a Ph.D. and BSc in physics from Imperial College, University of London, and an MSc in applied statistics from the University of Oxford, both in the U.K.
Anthony Duong, Ph.D. is a principal research scientist for materials science at Battelle. He has diverse expertise spanning coatings, nanotechnology, encapsulation, controlled release, and aerosols. Currently, Duong is developing concepts and technology for encapsulation and controlled release and is particularly interested in leveraging drug delivery technology from the pharmaceutical industry to meet consumer market needs in encapsulation and controlled release. Before joining Battelle in December 2013, Duong received his Ph.D. from Ohio State in chemical engineering. Subsequently, he carried out post-doctoral research in the Ohio State College of Pharmacy investigating drug delivery for immunology applications, including the development of vaccines and sustained release antimicrobials. Duong’s dissertation focused on processing and production of nanoparticles for drug delivery, imaging, and in-vitro cell detection and manipulation. He has developed novel processes for encapsulating bioactive agents in pH sensitive biocompatible polymer microparticles and liposomes.