Sean Fenske, Sam Brusco, and Michael Barbella08.11.20
The top companies in the orthopedic technology space represent the majority of the market. Similarly, those firms receive the lion’s share of coverage about their devices. As such, this feature sets aside space specifically to highlight exciting organizations developing or marketing unique innovations to help bring them much needed attention. Following, find six companies ODT’s editors have deemed worthwhile to be featured.
Sean Fenske • Editor-in-Chief
Many facets of the orthopedic device market are ripe for disruption. There is absolutely something to be said for “tried and true” technology, which is likely best reflected in the more traditional knee and hip implants—the bread and butter of the industry. But the potential for new innovations within the space can bring excitement and the hope for better solutions. That’s the connection between the following two companies selected for this year’s entry on emerging firms within the orthopedic device industry. Technologically, they are disparate, but in terms of their respective missions to enhance the quality and efficiency of care, they are quite similar.
Superman’s X-Ray Vision
Minimally invasive surgery offers tremendous benefits regarding its impact on the patient and post-operative healing timelines. It does, however, create ergonomic issues for the surgeon as compared to completely open procedures where the physician looks down at the surgical field. Instead, the doctor often looks at a monitor off to the side of the OR table to see via a camera that’s inside the patient.
Augmedics helps to restore a more natural surgical posture to the surgeon. The augmented reality solution—the xvision Spine System—enables the doctor to see “through” the patient’s skin and muscle while performing a specific procedure, such as spinal surgery. Better still, the system is instrument agnostic, so the surgeon can still employ their preferred instrument set from any vendor they choose. The xvision is a visual/imaging solution.
“Augmedics’ mission is to give surgeons more control by creating technological advances that cater to their needs and fit within their workflow,” said Nissan Elimelech, founder and CEO of Augmedics. “xvision is our first product of many to follow that will revolutionize surgery, as it gives surgeons the information they need, directly within their working field of sight, to instill technological confidence in the surgical workflow and help them do their jobs as effectively and safely as possible.”
The firm received an early Christmas gift in 2019 when it announced just a couple days before the holiday it had gained U.S. Food and Drug Administration (FDA) 510(k) clearance. The firm subsequently launched the product to the market at the same time.
Then in June 2020, the organization announced the first successful procedure had been performed with the xvision Spine System. Johns Hopkins University surgeons in Baltimore employed the technology in a spinal fusion procedure.
“In essence, it allows almost an ‘x-ray vision’ when we look at the patient,” said Dr. Daniel Sciubba, a professor of neurosurgery who was one of the surgeons performing the procedure. “This makes the surgery so much safer and faster as now we can ‘see’ things beyond what is normally visualized by the average surgeon.”
Fusion’s New Look
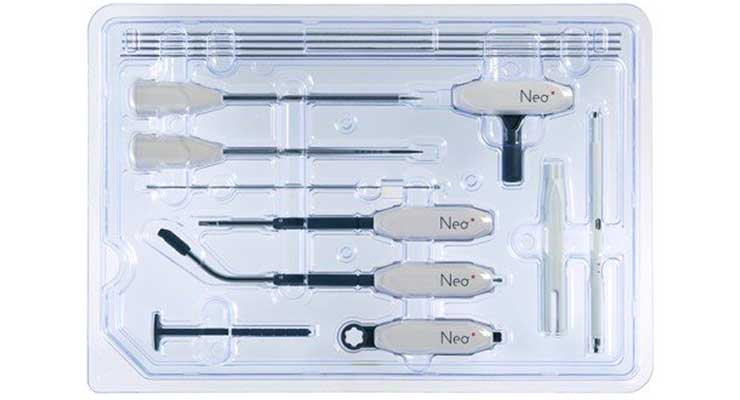
Spinal fusion is a relatively unchanged procedure, with incremental innovations typically representing the most substantial updates to the technologies. There are some, however, seeking to change that. Leveraging five single-use sterile instruments, 3D-printed titanium cages, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, Neo Medical is one such company attempting to change the paradigm.
“By starting from a blank page, basing our approach on science and published data, we have looked at and optimized every step of the surgery to give back the full control into the surgeon’s hands. With the objective to ensure the right correction, when required, and provide a fixation that can be perfectly adapted for every indication and most patients’ anatomies, we developed unique patented technologies resulting in a light, modular, and universal platform now replacing our current product offering,” Vincent Lefauconnier, CEO and founder of Neo Medical, explained in an interview with ODT.
The company is not simply producing a “better mousetrap” either. The new technologies require alterations to the surgical procedure itself. According to Lefauconnier, the new technique leads to a more efficient surgery, takes less time, and results in a lower infection rate and implant failure percentage. Neo Medical also offers a digital approach to training, which could be a more convenient option for physicians.
Further, since the instrumentation was developed to be single-use, there is no concern with cleaning and sterilization. Of course, with any disposable medical products, the question of sustainability emerges. Lefauconnier cited published studies where the environmental impact of the single-use instrumentation was compared to reusable tools.
“To compare the environmental impact of these different configurations, a comparative Life Cycle Assessment was performed. One of the key findings was the selected cleaning and sterilization process for reusable instruments is responsible for up to 90 percent of the greenhouse gas emissions and decides which system is advantageous from an environmental perspective. This study has shown a 75 percent reduction in the overall environmental footprint per surgery of our technology versus one of the legacy reusable systems,” said Lefauconnier.
The Neo Medical system may not prove to be the ideal offering for every case, but having alternatives to traditional fusion options is certainly advantageous.
Sam Brusco • Associate Editor
The orthopedics market is an interesting dichotomy. On one side, there are commodity knee and hip implants that have seen incremental improvement—mainly comprised of a few large companies controlling the market. On the other side, the market is made up of various smaller firms exploring ways to attack unmet clinical needs in the spine, small bone, and trauma markets. There is generally much more room for market share in these areas.
But even the high-growth markets are becoming crowded, so finding a way to differentiate from the many competitors is a must. Some firms experiment with innovative materials to enhance an orthopedic implant’s performance. Some employ a newer manufacturing method to boost production speed and reduce cost. Some even utilize a different business model to enhance value for customers. The following two orthopedic firms have used a mixture of these strategies to find success.
Set It and Forget It
San Diego-based Xenco Medical was founded in 2011 to replace traditional metal surgical systems and their cost-prohibitive manufacturing process with the first disposable, composite polymer spinal surgery systems.
The company’s SET Technology is a highly reinforced, composite polymer with durability gained from high interfacial bond strength. A highly durable fibrous matrix makes the implants able to withstand very high loads of force. The patient-specific spinal implant systems minimize risk of infection and cross contamination. The SET Technology portfolio includes a cervical interbody system; lumbar interbodies for ALIF, PLIF, and TLIF; and a pedicle screw system.
Last May, Xenco launched interactive vending machines to house, track, and dispense its spine surgery implants and instruments, a first according to the company. The vending machines are Wi-Fi enabled and use an elevator-based system to retrieve the sterile-packaged product. Users interact with a large, touchscreen interface to select the desired products and send real-time alerts to Xenco headquarters. The vending machines also feature virtual tutorials for Xenco products with a virtual assistant named Ezra.
“Extremely encouraged by the market’s response to our disposable, composite polymer systems, we’ve employed advanced, logistics-based technologies to deepen the impact our disposable systems make in streamlining the healthcare supply chain,” Xenco Medical founder and CEO Jason Haider told the press.
This past June, the company introduced what it claims are the first injection-molded titanium foam spinal implants—the CancelleX porous titanium lumbar interbodies—pre-attached to disposable, composite polymer instruments. Each implant features high compressive strength and interconnected porosity to promote bone apposition and facilitate vascularization. The pores draw in fresh blood, nourishing growing bone as it fuses to the implant.
“It’s almost like an optical illusion,” Haider told The San Diego Union-Tribune. “You hold [the implant] and it feels very lightweight, almost like Styrofoam.”
Fixer-Upper
Maple Grove, Minn.-based Conventus was formed in 2008 by a team of medical professionals seeking less invasive ways to treat challenging periarticular fractures. Using advanced nitinol technology, the team developed a platform technology to allow robust fixation and reliable repairs.
The result was the Conventus Cage, a less-invasive, self expanding fragment-specific system for proximal humerus, distal radius, and promixal radius fractures that matches the stability of locking plates and has a less invasive path of pinning. The Cage, cleared by the FDA in September 2015, expands from a cannulated delivery system to lock within the implant site, and provides increase in subchondral surface area compared to locking plates or rods, according to the firm. The Cage also creates versatile angle-stable fragment fixation from virtually any angle, and robust fixation and stability even in compromised bone. Patients with fractured wrists, elbows, and shoulders can avoid joint replacement by using the Conventus Cage.
Making its first step into lower extremity orthopedics, the firm acquired IntraFuse in December, gaining the FDA-cleared FlexThread intramedullary implants for minimally invasive fibula fracture repair.
“These products complement our intramedullary Cage products for large bone peri-articular fractures,” commented Rick Epstein, who was appointed CEO of Conventus last June. “…this acquisition is a first step of what will be an aggressive transformation from a single product company into a formidable orthopedic supplier.”
Flower Orthopedics was welcomed to the fold in April, bringing to Conventus sterile-packaged anatomic foot and ankle implants, allografts, and diabetic wound care solutions for orthopedic surgeons and podiatrists. The newly gained “Ready-for-Surgery” FlowerCube system removes expensive set processing, letting surgical facilities complete back-to-back cases and maximize resources.
Michael Barbella • Managing Editor
The great ones—the specialists with exceptional skills, instinct, and foresight—make it look easy. But even the great ones make mistakes once in a while.
Not surprising, considering all the complexities associated with total hip replacement (THR). From the initial incision (big or small) to the closing stitch, any number of issues can arise, from improper fit (too tight or too loose), cup misalignment, and implant conversion, to leg length restoration and femoral bone reconstruction.
“This is all about confidence...” California orthopedic surgeon Michael M. Karch quipped last spring. “Taking multiple X-rays to try and match the leg length of one leg to the other and roughly finding the appropriate hip muscle tension are a thing of the past.”
The not-so-distant past, actually. Computer-assisted surgery—navigation technology, specifically—has propelled hip replacements into the 21st century and helped improve both patient and procedural outcomes.
“It is unbelievable technology,” Karch said of an FDA-approved computer navigation system. “This navigation tool helps me quickly identify the exact position that hip components need to be placed in the operating room. If I want to put the hip component in the ideal position of 40 degrees relative to the floor and 20 degrees facing forward, I get 40 and 20 every time. There is no guesswork. I walk out of the operating room knowing we have done the absolute best for every patient, every time.”
THR’s future has finally arrived.
Look, No Image
Many total hip replacement navigation systems use fluoroscopic imaging, CTs, or MRIs to pre-plan the surgery and accurately position the artificial joint. NaviSwiss AG’s technology, however, needs no such visuals to offer precise data on cup alignment, leg length, and offset. Its THR application supports all surgical approaches, and displays combined anteversion (the sum of acetabular anteversion and femoral antetorsion).
“The NaviSwiss system is unique in both form and function. As a result of developing our own components, our core technology is smaller, lighter, and more robust,” CEO Jan Stiffer explained in a news release. “We reduce the cost of computer-assisted hip replacement and empower surgeons with more information specific to a patient’s anatomy. These are compelling features to the ever-developing U.S. medical industry.”
Naviswiss’s navigation system consists of a handheld device and three miniature precision tracking tags. One tag is attached to the pelvis and acts as a fixed point for cup navigation while another is seated on the cup impactor via a magnet. A removable third tag, meanwhile, is affixed to the femur to measure leg length and offset.
The system reportedly can accurately detect leg length changes to the millimeter, helping reduce patient dissatisfaction and significantly mitigating the risk of chronic low back pain caused by leg length discrepancy.
Naviswiss’s THR application won Japanese regulatory approval late last year and FDA approval in June. The system is already marketed in Europe, Australia, and Israel. Late last fall, the Swiss firm named a chief commercial officer to spearhead its U.S. efforts and opened an American headquarters in Denver.
“There has been a need in the U.S. for a simple, cost-effective solution for navigated hip replacements,” chief commercial officer Daniel Moore said upon the FDA’s approval of the system. “Where technology has been quite common in knee replacements in recent years, there has still been a technology gap in hip replacements. This is largely due to the complexity and cost of previous technologies. We can now offer a simple, imageless solution to patients and healthcare providers with the potential of reducing the rate of revisions as well as reducing radiation exposure.”
The Phantom (Distortion) Menace
Orthopedics is a science of precision. The human musculoskeletal system is a biological wonder, comprised of a complex network of bones, cartilage, muscles, tendons, ligaments, joints, and connective tissue that allow for movement. Treating and/or replacing any of these network links requires near flawless accuracy for long-term success.
Yet, precision is a rarity in modern medicine. Consider, for example, the paradox presented by joint replacements: These procedures require a high level of accuracy but often use pre- and intra-operative fluoroscopic imaging to determine implant positioning and fit. Such images (fluoroscopic X-rays), though, are not the best or most precise measurement tool, as they are uncorrelated, geometrically distorted, and have a small field of view.
OrthoGrid Systems Inc. has developed technology that corrects for fluoroscopic distortion in multiple procedures, including the direct anterior approach in total hip arthroplasty. The Salt Lake City company’s PhantomMSK System helps restore native hip biomechanics with alignment considerations for leg length, femoral offset, cup inclination, femoral abduction, pelvic tilt, and pelvic obliquity.
PhantomMSK is a software-based device that can be run on commercial “off-the-shelf” systems that meet minimum performance requirements (PCs, keyboards, touchscreen monitors, a mouse, etc.).
Operating the PhantomMSK System is simple: A fluoroscopic image is acquired from a C-arm and displayed outside the sterile field, where image analysis tools are used at the surgeon’s discretion.
“I think if you’re going to do an accurate hip replacement, if you’re going to do something precise and you’re aiming for perfection every time, I think it’s an absolute necessity,” Alex Brothers, M.D., a University of Utah physician, said of PhantomMSK. “I think correcting for distortion is a key part of making sure that everything goes as perfectly as possible.”
PhantomMSK supposedly is the only intra-operative alignment technology on the market to correct image distortion related to external electromagnetic interference during orthopedic surgeries.
Sean Fenske • Editor-in-Chief
Many facets of the orthopedic device market are ripe for disruption. There is absolutely something to be said for “tried and true” technology, which is likely best reflected in the more traditional knee and hip implants—the bread and butter of the industry. But the potential for new innovations within the space can bring excitement and the hope for better solutions. That’s the connection between the following two companies selected for this year’s entry on emerging firms within the orthopedic device industry. Technologically, they are disparate, but in terms of their respective missions to enhance the quality and efficiency of care, they are quite similar.
Superman’s X-Ray Vision
Minimally invasive surgery offers tremendous benefits regarding its impact on the patient and post-operative healing timelines. It does, however, create ergonomic issues for the surgeon as compared to completely open procedures where the physician looks down at the surgical field. Instead, the doctor often looks at a monitor off to the side of the OR table to see via a camera that’s inside the patient.
Augmedics helps to restore a more natural surgical posture to the surgeon. The augmented reality solution—the xvision Spine System—enables the doctor to see “through” the patient’s skin and muscle while performing a specific procedure, such as spinal surgery. Better still, the system is instrument agnostic, so the surgeon can still employ their preferred instrument set from any vendor they choose. The xvision is a visual/imaging solution.
“Augmedics’ mission is to give surgeons more control by creating technological advances that cater to their needs and fit within their workflow,” said Nissan Elimelech, founder and CEO of Augmedics. “xvision is our first product of many to follow that will revolutionize surgery, as it gives surgeons the information they need, directly within their working field of sight, to instill technological confidence in the surgical workflow and help them do their jobs as effectively and safely as possible.”
The firm received an early Christmas gift in 2019 when it announced just a couple days before the holiday it had gained U.S. Food and Drug Administration (FDA) 510(k) clearance. The firm subsequently launched the product to the market at the same time.
Then in June 2020, the organization announced the first successful procedure had been performed with the xvision Spine System. Johns Hopkins University surgeons in Baltimore employed the technology in a spinal fusion procedure.
“In essence, it allows almost an ‘x-ray vision’ when we look at the patient,” said Dr. Daniel Sciubba, a professor of neurosurgery who was one of the surgeons performing the procedure. “This makes the surgery so much safer and faster as now we can ‘see’ things beyond what is normally visualized by the average surgeon.”
Fusion’s New Look
Spinal fusion is a relatively unchanged procedure, with incremental innovations typically representing the most substantial updates to the technologies. There are some, however, seeking to change that. Leveraging five single-use sterile instruments, 3D-printed titanium cages, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, Neo Medical is one such company attempting to change the paradigm.
“By starting from a blank page, basing our approach on science and published data, we have looked at and optimized every step of the surgery to give back the full control into the surgeon’s hands. With the objective to ensure the right correction, when required, and provide a fixation that can be perfectly adapted for every indication and most patients’ anatomies, we developed unique patented technologies resulting in a light, modular, and universal platform now replacing our current product offering,” Vincent Lefauconnier, CEO and founder of Neo Medical, explained in an interview with ODT.
The company is not simply producing a “better mousetrap” either. The new technologies require alterations to the surgical procedure itself. According to Lefauconnier, the new technique leads to a more efficient surgery, takes less time, and results in a lower infection rate and implant failure percentage. Neo Medical also offers a digital approach to training, which could be a more convenient option for physicians.
Further, since the instrumentation was developed to be single-use, there is no concern with cleaning and sterilization. Of course, with any disposable medical products, the question of sustainability emerges. Lefauconnier cited published studies where the environmental impact of the single-use instrumentation was compared to reusable tools.
“To compare the environmental impact of these different configurations, a comparative Life Cycle Assessment was performed. One of the key findings was the selected cleaning and sterilization process for reusable instruments is responsible for up to 90 percent of the greenhouse gas emissions and decides which system is advantageous from an environmental perspective. This study has shown a 75 percent reduction in the overall environmental footprint per surgery of our technology versus one of the legacy reusable systems,” said Lefauconnier.
The Neo Medical system may not prove to be the ideal offering for every case, but having alternatives to traditional fusion options is certainly advantageous.
Sam Brusco • Associate Editor
The orthopedics market is an interesting dichotomy. On one side, there are commodity knee and hip implants that have seen incremental improvement—mainly comprised of a few large companies controlling the market. On the other side, the market is made up of various smaller firms exploring ways to attack unmet clinical needs in the spine, small bone, and trauma markets. There is generally much more room for market share in these areas.
But even the high-growth markets are becoming crowded, so finding a way to differentiate from the many competitors is a must. Some firms experiment with innovative materials to enhance an orthopedic implant’s performance. Some employ a newer manufacturing method to boost production speed and reduce cost. Some even utilize a different business model to enhance value for customers. The following two orthopedic firms have used a mixture of these strategies to find success.
Set It and Forget It
San Diego-based Xenco Medical was founded in 2011 to replace traditional metal surgical systems and their cost-prohibitive manufacturing process with the first disposable, composite polymer spinal surgery systems.
The company’s SET Technology is a highly reinforced, composite polymer with durability gained from high interfacial bond strength. A highly durable fibrous matrix makes the implants able to withstand very high loads of force. The patient-specific spinal implant systems minimize risk of infection and cross contamination. The SET Technology portfolio includes a cervical interbody system; lumbar interbodies for ALIF, PLIF, and TLIF; and a pedicle screw system.
Last May, Xenco launched interactive vending machines to house, track, and dispense its spine surgery implants and instruments, a first according to the company. The vending machines are Wi-Fi enabled and use an elevator-based system to retrieve the sterile-packaged product. Users interact with a large, touchscreen interface to select the desired products and send real-time alerts to Xenco headquarters. The vending machines also feature virtual tutorials for Xenco products with a virtual assistant named Ezra.
“Extremely encouraged by the market’s response to our disposable, composite polymer systems, we’ve employed advanced, logistics-based technologies to deepen the impact our disposable systems make in streamlining the healthcare supply chain,” Xenco Medical founder and CEO Jason Haider told the press.
This past June, the company introduced what it claims are the first injection-molded titanium foam spinal implants—the CancelleX porous titanium lumbar interbodies—pre-attached to disposable, composite polymer instruments. Each implant features high compressive strength and interconnected porosity to promote bone apposition and facilitate vascularization. The pores draw in fresh blood, nourishing growing bone as it fuses to the implant.
“It’s almost like an optical illusion,” Haider told The San Diego Union-Tribune. “You hold [the implant] and it feels very lightweight, almost like Styrofoam.”
Fixer-Upper
Maple Grove, Minn.-based Conventus was formed in 2008 by a team of medical professionals seeking less invasive ways to treat challenging periarticular fractures. Using advanced nitinol technology, the team developed a platform technology to allow robust fixation and reliable repairs.
The result was the Conventus Cage, a less-invasive, self expanding fragment-specific system for proximal humerus, distal radius, and promixal radius fractures that matches the stability of locking plates and has a less invasive path of pinning. The Cage, cleared by the FDA in September 2015, expands from a cannulated delivery system to lock within the implant site, and provides increase in subchondral surface area compared to locking plates or rods, according to the firm. The Cage also creates versatile angle-stable fragment fixation from virtually any angle, and robust fixation and stability even in compromised bone. Patients with fractured wrists, elbows, and shoulders can avoid joint replacement by using the Conventus Cage.
Making its first step into lower extremity orthopedics, the firm acquired IntraFuse in December, gaining the FDA-cleared FlexThread intramedullary implants for minimally invasive fibula fracture repair.
“These products complement our intramedullary Cage products for large bone peri-articular fractures,” commented Rick Epstein, who was appointed CEO of Conventus last June. “…this acquisition is a first step of what will be an aggressive transformation from a single product company into a formidable orthopedic supplier.”
Flower Orthopedics was welcomed to the fold in April, bringing to Conventus sterile-packaged anatomic foot and ankle implants, allografts, and diabetic wound care solutions for orthopedic surgeons and podiatrists. The newly gained “Ready-for-Surgery” FlowerCube system removes expensive set processing, letting surgical facilities complete back-to-back cases and maximize resources.
Michael Barbella • Managing Editor
The great ones—the specialists with exceptional skills, instinct, and foresight—make it look easy. But even the great ones make mistakes once in a while.
Not surprising, considering all the complexities associated with total hip replacement (THR). From the initial incision (big or small) to the closing stitch, any number of issues can arise, from improper fit (too tight or too loose), cup misalignment, and implant conversion, to leg length restoration and femoral bone reconstruction.
“This is all about confidence...” California orthopedic surgeon Michael M. Karch quipped last spring. “Taking multiple X-rays to try and match the leg length of one leg to the other and roughly finding the appropriate hip muscle tension are a thing of the past.”
The not-so-distant past, actually. Computer-assisted surgery—navigation technology, specifically—has propelled hip replacements into the 21st century and helped improve both patient and procedural outcomes.
“It is unbelievable technology,” Karch said of an FDA-approved computer navigation system. “This navigation tool helps me quickly identify the exact position that hip components need to be placed in the operating room. If I want to put the hip component in the ideal position of 40 degrees relative to the floor and 20 degrees facing forward, I get 40 and 20 every time. There is no guesswork. I walk out of the operating room knowing we have done the absolute best for every patient, every time.”
THR’s future has finally arrived.
Look, No Image
Many total hip replacement navigation systems use fluoroscopic imaging, CTs, or MRIs to pre-plan the surgery and accurately position the artificial joint. NaviSwiss AG’s technology, however, needs no such visuals to offer precise data on cup alignment, leg length, and offset. Its THR application supports all surgical approaches, and displays combined anteversion (the sum of acetabular anteversion and femoral antetorsion).
“The NaviSwiss system is unique in both form and function. As a result of developing our own components, our core technology is smaller, lighter, and more robust,” CEO Jan Stiffer explained in a news release. “We reduce the cost of computer-assisted hip replacement and empower surgeons with more information specific to a patient’s anatomy. These are compelling features to the ever-developing U.S. medical industry.”
Naviswiss’s navigation system consists of a handheld device and three miniature precision tracking tags. One tag is attached to the pelvis and acts as a fixed point for cup navigation while another is seated on the cup impactor via a magnet. A removable third tag, meanwhile, is affixed to the femur to measure leg length and offset.
The system reportedly can accurately detect leg length changes to the millimeter, helping reduce patient dissatisfaction and significantly mitigating the risk of chronic low back pain caused by leg length discrepancy.
Naviswiss’s THR application won Japanese regulatory approval late last year and FDA approval in June. The system is already marketed in Europe, Australia, and Israel. Late last fall, the Swiss firm named a chief commercial officer to spearhead its U.S. efforts and opened an American headquarters in Denver.
“There has been a need in the U.S. for a simple, cost-effective solution for navigated hip replacements,” chief commercial officer Daniel Moore said upon the FDA’s approval of the system. “Where technology has been quite common in knee replacements in recent years, there has still been a technology gap in hip replacements. This is largely due to the complexity and cost of previous technologies. We can now offer a simple, imageless solution to patients and healthcare providers with the potential of reducing the rate of revisions as well as reducing radiation exposure.”
The Phantom (Distortion) Menace
Orthopedics is a science of precision. The human musculoskeletal system is a biological wonder, comprised of a complex network of bones, cartilage, muscles, tendons, ligaments, joints, and connective tissue that allow for movement. Treating and/or replacing any of these network links requires near flawless accuracy for long-term success.
Yet, precision is a rarity in modern medicine. Consider, for example, the paradox presented by joint replacements: These procedures require a high level of accuracy but often use pre- and intra-operative fluoroscopic imaging to determine implant positioning and fit. Such images (fluoroscopic X-rays), though, are not the best or most precise measurement tool, as they are uncorrelated, geometrically distorted, and have a small field of view.
OrthoGrid Systems Inc. has developed technology that corrects for fluoroscopic distortion in multiple procedures, including the direct anterior approach in total hip arthroplasty. The Salt Lake City company’s PhantomMSK System helps restore native hip biomechanics with alignment considerations for leg length, femoral offset, cup inclination, femoral abduction, pelvic tilt, and pelvic obliquity.
PhantomMSK is a software-based device that can be run on commercial “off-the-shelf” systems that meet minimum performance requirements (PCs, keyboards, touchscreen monitors, a mouse, etc.).
Operating the PhantomMSK System is simple: A fluoroscopic image is acquired from a C-arm and displayed outside the sterile field, where image analysis tools are used at the surgeon’s discretion.
“I think if you’re going to do an accurate hip replacement, if you’re going to do something precise and you’re aiming for perfection every time, I think it’s an absolute necessity,” Alex Brothers, M.D., a University of Utah physician, said of PhantomMSK. “I think correcting for distortion is a key part of making sure that everything goes as perfectly as possible.”
PhantomMSK supposedly is the only intra-operative alignment technology on the market to correct image distortion related to external electromagnetic interference during orthopedic surgeries.