Michael Barbella , Managing Editor09.14.21
It wasn’t the pain that bothered Lia Winter so much that day on the soccer field.
She figured the misery eminating from the back of her thigh would eventually abate. Considerably more concerning to the then-teenaged Winter was the length of time she’d be sidelined from her favorite sport.
Her concern was typical, given her age, dedication to her sport, and general lack of knowledge about hamstring tears. Based on the advice of a family friend, Winter treated her injury with platelet-rich plasma injections and salvaged part of her soccer season.
“I made a full recovery and was able to play in the last two games of the season,” she said in a recent National Inventors Hall of Fame interview. “That experience opened my eyes to the possibilities of medical innovation, and I decided to pursue a degree in biomedical engineering.”
While pursuing her degree at the University of Pittsburgh, Winter interned as an orthopedic product tester, measuring suture needle efficacy. In preparing grafts for mechanical testing, she learned that whipstitching is prone to human error. A running whipstitch—a.k.a., a quick running suture—often is used to close scalp wounds, C-section incisions, slits from numerous types of surgeries (heart, laparoscopic, etc.), and athletic injuries, particularly tendon and ligament tears.
The latter traumas are particularly susceptible to blunders: Up to 25 percent of tendon and ligament repair surgeries reportedly have failed, and most failures have been attributed to technical errors, research shows. These errors cost the U.S. healthcare system $2.5 billion annually, industry data indicate.
“I did a lot of R&D and product testing and was struck by how tedious and inconsistent the stitching they would do for surgeries was,” Winter told The Business Journal of Tri-Cities Tennessee/Virginia last March. “So, I came up with an idea to create a new needle to make it easier and faster for the surgeon to stitch.”
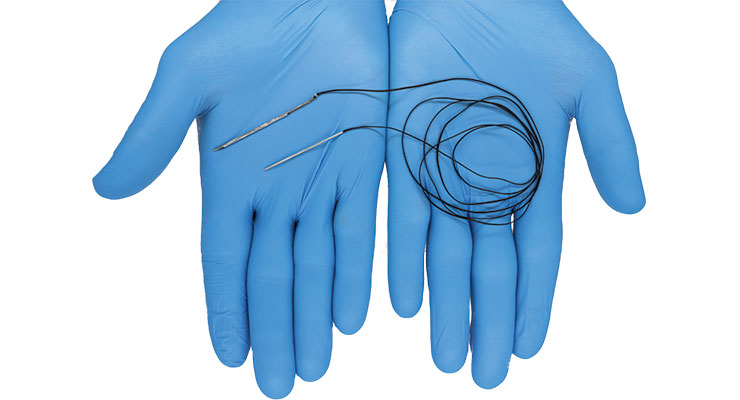
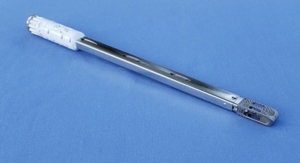
That new needle, EasyWhip, received U.S. Food and Drug Administration (FDA) 510(k) clearance in May. Designed in tandem with fellow intern and University of Tennessee-Knoxville MBA graduate Preston Dishner, EasyWhip is a patented two-part needle featuring a needle tip and connectable rod/insert. The two needle components are fixed to ends of a length of ultra-high strength orthopedic suture. They can be connected to create a continuous loop of suture and disconnected to create a straight length of suture, which facilitates faster, easier stitching and creation of patterns that cannot be made with conventional needles.
The EasyWhip is indicated for use in approximation and/or ligation of soft tissues, including the use of allograft tissue for orthopedic surgeries. It is ideal for repairing torn ligaments and tendons, like the anterior cruciate ligament (ACL), Achilles’ tendon, and distal biceps tendon.
“The beauty of EasyWhip is that it’s sophisticated, yet so simple. And it’s reproducible,” stated James Bradley, M.D., an orthopedic surgeon in Pittsburgh and team physician for the Pittsburgh Steelers. “Because of its configuration, there are three or four different stitch patterns that you can make with EasyWhip. Timing-wise, I’m much faster with this product than I am using a traditional whip stitch needle.”
The EasyWhip’s clearance is the first regulatory milestone for Winter Innovations Inc., the company Winter and Dishner (its chief operating officer) founded in 2018. Shortly before gaining FDA clearance this past spring, the company received a $256,000 National Science Foundation Small Business Innovation Research (SBIR) grant to conduct research and development (R&D) work on its two-part suturing needle technology.
In this newly funded project, the Winter Innovations’ team will create prototypes with varying characteristics, study the performance and usability of those prototypes in simulated use settings, and make comparisons to existing needle products currently used in orthopedic surgery. This project will allow Winter Innovations to advance the two-part needle technology toward commercialization in orthopedics and provide a basis for additional research of the needle design for other surgical applications that rely on stitching.
“FDA clearance is the biggest milestone we have achieved to date. We spent a lot of time in product development making sure that EasyWhip solves the problems we identified with orthopedic stitching to repair torn ligaments and tendons, like the anterior cruciate ligament,” Winter, founder and CEO of Winter Innovations, told the University of Tennessee-Knoxville press. “Now that we have the FDA clearance, we can start marketing the product and its associated speed and ease-of-use benefits to surgeons and hospitals. We are looking forward to the opportunity to launch EasyWhip and positively impact the lives of surgeons and patients.”
She figured the misery eminating from the back of her thigh would eventually abate. Considerably more concerning to the then-teenaged Winter was the length of time she’d be sidelined from her favorite sport.
Her concern was typical, given her age, dedication to her sport, and general lack of knowledge about hamstring tears. Based on the advice of a family friend, Winter treated her injury with platelet-rich plasma injections and salvaged part of her soccer season.
“I made a full recovery and was able to play in the last two games of the season,” she said in a recent National Inventors Hall of Fame interview. “That experience opened my eyes to the possibilities of medical innovation, and I decided to pursue a degree in biomedical engineering.”
While pursuing her degree at the University of Pittsburgh, Winter interned as an orthopedic product tester, measuring suture needle efficacy. In preparing grafts for mechanical testing, she learned that whipstitching is prone to human error. A running whipstitch—a.k.a., a quick running suture—often is used to close scalp wounds, C-section incisions, slits from numerous types of surgeries (heart, laparoscopic, etc.), and athletic injuries, particularly tendon and ligament tears.
The latter traumas are particularly susceptible to blunders: Up to 25 percent of tendon and ligament repair surgeries reportedly have failed, and most failures have been attributed to technical errors, research shows. These errors cost the U.S. healthcare system $2.5 billion annually, industry data indicate.
“I did a lot of R&D and product testing and was struck by how tedious and inconsistent the stitching they would do for surgeries was,” Winter told The Business Journal of Tri-Cities Tennessee/Virginia last March. “So, I came up with an idea to create a new needle to make it easier and faster for the surgeon to stitch.”
That new needle, EasyWhip, received U.S. Food and Drug Administration (FDA) 510(k) clearance in May. Designed in tandem with fellow intern and University of Tennessee-Knoxville MBA graduate Preston Dishner, EasyWhip is a patented two-part needle featuring a needle tip and connectable rod/insert. The two needle components are fixed to ends of a length of ultra-high strength orthopedic suture. They can be connected to create a continuous loop of suture and disconnected to create a straight length of suture, which facilitates faster, easier stitching and creation of patterns that cannot be made with conventional needles.
The EasyWhip is indicated for use in approximation and/or ligation of soft tissues, including the use of allograft tissue for orthopedic surgeries. It is ideal for repairing torn ligaments and tendons, like the anterior cruciate ligament (ACL), Achilles’ tendon, and distal biceps tendon.
“The beauty of EasyWhip is that it’s sophisticated, yet so simple. And it’s reproducible,” stated James Bradley, M.D., an orthopedic surgeon in Pittsburgh and team physician for the Pittsburgh Steelers. “Because of its configuration, there are three or four different stitch patterns that you can make with EasyWhip. Timing-wise, I’m much faster with this product than I am using a traditional whip stitch needle.”
The EasyWhip’s clearance is the first regulatory milestone for Winter Innovations Inc., the company Winter and Dishner (its chief operating officer) founded in 2018. Shortly before gaining FDA clearance this past spring, the company received a $256,000 National Science Foundation Small Business Innovation Research (SBIR) grant to conduct research and development (R&D) work on its two-part suturing needle technology.
In this newly funded project, the Winter Innovations’ team will create prototypes with varying characteristics, study the performance and usability of those prototypes in simulated use settings, and make comparisons to existing needle products currently used in orthopedic surgery. This project will allow Winter Innovations to advance the two-part needle technology toward commercialization in orthopedics and provide a basis for additional research of the needle design for other surgical applications that rely on stitching.
“FDA clearance is the biggest milestone we have achieved to date. We spent a lot of time in product development making sure that EasyWhip solves the problems we identified with orthopedic stitching to repair torn ligaments and tendons, like the anterior cruciate ligament,” Winter, founder and CEO of Winter Innovations, told the University of Tennessee-Knoxville press. “Now that we have the FDA clearance, we can start marketing the product and its associated speed and ease-of-use benefits to surgeons and hospitals. We are looking forward to the opportunity to launch EasyWhip and positively impact the lives of surgeons and patients.”