Business Wire06.19.17
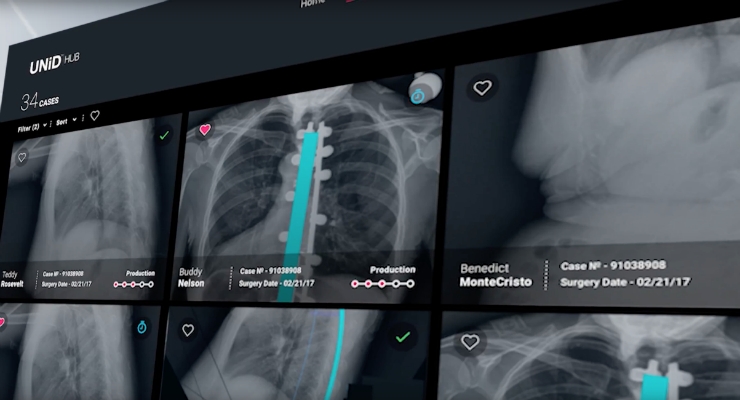
The Medicrea Group, pioneering the convergence of healthcare IT and next-generation, outcome-centered device design and manufacturing with UNiD ASI technology, announced that it has received 510(k) Clearance from the Food & Drug Administration for UNiD HUB, a data-driven digital portal for the company’s Adaptive Spine Intelligence.
“It is our belief that data science and analytics are critical components of improved patient outcomes and efficiencies. For that reason, Medicrea has stepped into a space unoccupied by traditional device manufacturers: Software development and Artificial Intelligence,” stated Denys Sournac, president and CEO of Medicrea. “The UNiD HUB represents Medicrea’s ability to lead the Spine industry with breakthrough innovations by offering a unique forward-thinking and holistic approach to personalized spinal surgery.”
The UNiD HUB is designed to support the surgeon workflow, identify tendencies and correlations, and build predictive modeling to drive intelligent strategic decisions and create personalized implant solutions for surgery. The UNiD HUB software also serves to enhance the existing proprietary IT utilized by Medicrea’s UNiD ASI platform for digital surgical planning to create a seamless communication channel between the Company’s UNiD LAB biomedical engineers and Surgeon users to deliver UNiD TEK, patient-specific spinal implants manufactured by Medicrea through proprietary rod bending and 3D printing techniques.
The digital communication portal opened with the introduction of UNiD HUB instantly creates a sticky, user-friendly environment for surgeons to track and manage their open cases in both snapshot and detailed views, access their complete history with post-operative analyses and dialogue with a dedicated biomedical engineer in real time. Future functionalities will become available alongside the software’s wide release planned in October of 2017, for Eurospine in Dublin, Ireland on the 11-13 October and at the annual meeting of the North American Spine Society, held 25-28 October in Orlando, Fla.
Sournac continued, “As a media tool for surgeons, we have received very positive feedback from our UNiD ASI customers who have seen beta versions of the software and we are now ready to make the UNiD HUB digital portal immediately available to them. The software integrates well with their clinical habits and enhances their experience with UNiD ASI technology.” Sournac added, “This new generation of software will be more than a communication tool for surgeons—it will grant surgeons access to the same data analytics and machine learning technology that is curated by our data scientists to use the UNiD HUB as an unparalleled research engine and networking platform.”
UNiD HUB has been developed in desktop and mobile applications and is accessible from medical offices as well as on the go.
“It is our belief that data science and analytics are critical components of improved patient outcomes and efficiencies. For that reason, Medicrea has stepped into a space unoccupied by traditional device manufacturers: Software development and Artificial Intelligence,” stated Denys Sournac, president and CEO of Medicrea. “The UNiD HUB represents Medicrea’s ability to lead the Spine industry with breakthrough innovations by offering a unique forward-thinking and holistic approach to personalized spinal surgery.”
The UNiD HUB is designed to support the surgeon workflow, identify tendencies and correlations, and build predictive modeling to drive intelligent strategic decisions and create personalized implant solutions for surgery. The UNiD HUB software also serves to enhance the existing proprietary IT utilized by Medicrea’s UNiD ASI platform for digital surgical planning to create a seamless communication channel between the Company’s UNiD LAB biomedical engineers and Surgeon users to deliver UNiD TEK, patient-specific spinal implants manufactured by Medicrea through proprietary rod bending and 3D printing techniques.
The digital communication portal opened with the introduction of UNiD HUB instantly creates a sticky, user-friendly environment for surgeons to track and manage their open cases in both snapshot and detailed views, access their complete history with post-operative analyses and dialogue with a dedicated biomedical engineer in real time. Future functionalities will become available alongside the software’s wide release planned in October of 2017, for Eurospine in Dublin, Ireland on the 11-13 October and at the annual meeting of the North American Spine Society, held 25-28 October in Orlando, Fla.
Sournac continued, “As a media tool for surgeons, we have received very positive feedback from our UNiD ASI customers who have seen beta versions of the software and we are now ready to make the UNiD HUB digital portal immediately available to them. The software integrates well with their clinical habits and enhances their experience with UNiD ASI technology.” Sournac added, “This new generation of software will be more than a communication tool for surgeons—it will grant surgeons access to the same data analytics and machine learning technology that is curated by our data scientists to use the UNiD HUB as an unparalleled research engine and networking platform.”
UNiD HUB has been developed in desktop and mobile applications and is accessible from medical offices as well as on the go.