Business Wire11.08.18
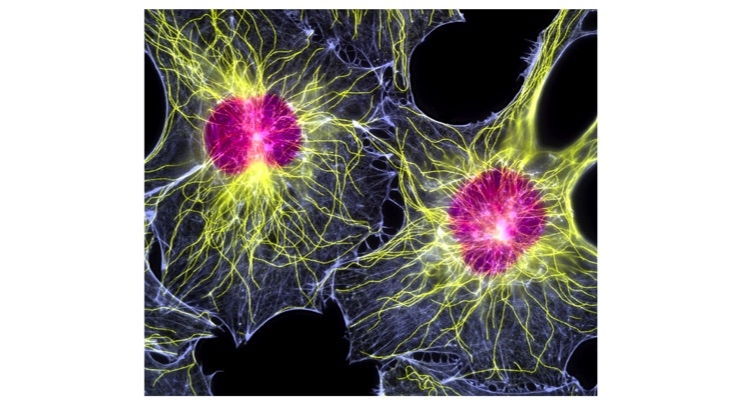
SpinalCyte LLC, a regenerative medicine company focused on regrowth of the spinal disc using Human Dermal Fibroblasts (HDFs), has been issued new patents in Hong Kong and Europe. The company’s intellectual property in spine treatments now includes 35 U.S. and foreign patents issued with 41 patents pending, which provide an intellectual property estate for its lead product, CybroCell. The company’s positive results from its completed clinical trial and strong patent portfolio continue to spur interest among biotech investors for licensing and partnership opportunities.
“These new patents add to our portfolio and bolster the international protections for our fibroblast technology,” said Pete O’Heeron, CEO of SpinalCyte. “SpinalCyte is at the forefront of human dermal fibroblast cell therapy, specifically in disc degeneration. We hold all intellectual property related to HDF treatment for degenerative disc disease (DDD), positioning us as the front-runner in this emerging sector of cell therapies.”
The technologies described in Hong Kong Patent No. HK1197832, “Dermal Fibroblasts for Treatment of Degenerative Disc Disease,” and European Patent No. 3146939, “Composition for Repair of Cartilage Using an In Vivo Bioreactor,” related to generating chondrocyte-like cells and applying them within the spinal disc space. Additional claims surround the paracrine benefit of introducing fibroblasts to the disc environment.
The new patents follow positive clinical trial results of CybroCell which show sustained pain relief in the treatment of DDD at 12 months.
Degenerative disc disease is a condition in which a patient’s spinal disc breaks down and can begin to collapse. It is estimated that 85 percent of people over the age of 50 have evidence of disc degeneration and over 1.3 million procedures a year are performed to treat the disease. The most common treatments for patients with DDD are either discectomy or spinal fusion. Discectomy is the partial or full removal of the degenerated disc to decompress and relieve the nervous system but can cause long term spinal pain. In a spinal fusion procedure, the entire disc is removed and the two adjacent vertebrae are fused together. It often increases strain on the adjacent discs and surrounding tissues leading to further degeneration.
CybroCell is the first off-the-shelf allogenic human dermal fibroblast (HDF) product for the treatment of degenerative disc disease. SpinalCyte’s Phase 1/Phase 2 clinical trial for injected human dermal fibroblasts in the treatment of DDD demonstrated after 12 months, patients who were injected with CybroCell had sustained improvement in pain relief and increased back mobility.
Based in Houston, Texas, SpinalCyte LLC is a regenerative medicine company developing a solution for spinal disc regeneration using human dermal fibroblasts. Currently, SpinalCyte holds 35 U.S. and international issued patents and has filed for an additional 41 patents pending. SpinalCyte holds 110 U.S. and International Patents pending and issued across a variety of clinical pathways, including disc degeneration, cancer, diabetes, liver failure and heart failure. The company is funded entirely by angel investors.
“These new patents add to our portfolio and bolster the international protections for our fibroblast technology,” said Pete O’Heeron, CEO of SpinalCyte. “SpinalCyte is at the forefront of human dermal fibroblast cell therapy, specifically in disc degeneration. We hold all intellectual property related to HDF treatment for degenerative disc disease (DDD), positioning us as the front-runner in this emerging sector of cell therapies.”
The technologies described in Hong Kong Patent No. HK1197832, “Dermal Fibroblasts for Treatment of Degenerative Disc Disease,” and European Patent No. 3146939, “Composition for Repair of Cartilage Using an In Vivo Bioreactor,” related to generating chondrocyte-like cells and applying them within the spinal disc space. Additional claims surround the paracrine benefit of introducing fibroblasts to the disc environment.
The new patents follow positive clinical trial results of CybroCell which show sustained pain relief in the treatment of DDD at 12 months.
Degenerative disc disease is a condition in which a patient’s spinal disc breaks down and can begin to collapse. It is estimated that 85 percent of people over the age of 50 have evidence of disc degeneration and over 1.3 million procedures a year are performed to treat the disease. The most common treatments for patients with DDD are either discectomy or spinal fusion. Discectomy is the partial or full removal of the degenerated disc to decompress and relieve the nervous system but can cause long term spinal pain. In a spinal fusion procedure, the entire disc is removed and the two adjacent vertebrae are fused together. It often increases strain on the adjacent discs and surrounding tissues leading to further degeneration.
CybroCell is the first off-the-shelf allogenic human dermal fibroblast (HDF) product for the treatment of degenerative disc disease. SpinalCyte’s Phase 1/Phase 2 clinical trial for injected human dermal fibroblasts in the treatment of DDD demonstrated after 12 months, patients who were injected with CybroCell had sustained improvement in pain relief and increased back mobility.
Based in Houston, Texas, SpinalCyte LLC is a regenerative medicine company developing a solution for spinal disc regeneration using human dermal fibroblasts. Currently, SpinalCyte holds 35 U.S. and international issued patents and has filed for an additional 41 patents pending. SpinalCyte holds 110 U.S. and International Patents pending and issued across a variety of clinical pathways, including disc degeneration, cancer, diabetes, liver failure and heart failure. The company is funded entirely by angel investors.