Medacta07.29.20
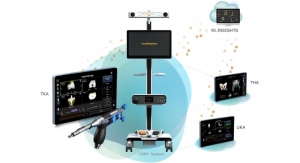
Medacta has received clearance from the FDA for its NextAR Augmented Reality based surgical platform for total knee arthroplasty (TKA) procedures.
The NextAR TKA is the first application of a new platform technology, which will be extended to hip, shoulder and spine procedures, designed and engineered with artificial intelligence and machine learning that make pre-operative CT-based planning and analysis efficient and precise.
The NextAR TKA Application is designed to improve efficiency and precision in total knee replacement and deliver personalized planning, with low upfront capital investment required by clinics and hospitals, as well as economic benefits to the healthcare system through OR efficiency and low cost per procedure. Medacta believes that this new platform will be an optimal solution particularly for U.S. ambulatory surgery centers (ASCs), which provide same-day surgical care.
The Augmented Reality glasses provided with the NextAR Platform allow the surgeon to visualize surgical actions and information in real-time, directly on the operative field. This improves the user experience and helps the surgeon remain focused on the patient at all times.
“With enhanced visualization via augmented reality, the NextAR Platform is an innovative and efficient tool for ultra-precise, patient-specific treatment,” said Dr Michael McAuliffe (MBBS FRACS), an orthopedic surgeon at St Andrew’s Ipswich Hospital in Queensland, Australia, and a member of the expert surgeon panel that Medacta collaborated with to develop the NextAR TKA Application. “With the NextAR TKA Application, we are able to track 3D soft tissue behavior during a surgery in real time. This functionality is an exciting advancement from traditional computer-assisted or robotic-assisted surgical systems that only track the relative movement of the knee bones, not the actual soft tissue.”
The NextAR TKA Application, with a precise reconstruction of the patient bone morphology, allows direct tracking of the collateral ligaments and a 3D analysis of soft tissue behavior throughout the whole range of motion during surgery, bringing patient-specific ligament balancing to the next level.
NextAR TS
In addition to advanced planning tools, Medacta has developed the NextAR TS, an infrared single-use tracking system that has the potential to vastly improve surgery efficiency while helping surgeons execute pre-op plans. Particularly in today’s environment, as surgeons and health systems look to recover surgeries that were deferred during the COVID-19 pandemic, efficiency in the operating room will become even more crucial than before. The NextAR Platform with its Single-Use Infrared Tracking System has the goal to meet these new levels of market demand.
The NextAR TKA works in conjunction with the Medacta GMK Sphere Medially Stabilized Knee implant, which has been proven to facilitate restoration of natural patient-specific kinematics.
Francesco Siccardi, CEO of Medacta, commented, “Patients and surgeons are attracted by improved outcomes and innovation, the NextAR Platform is one Medacta answer to the current race in orthopedic technology and I am very excited about this milestone achievement. Requiring a very limited investment in capital equipment, the NextAR Platform perfectly represents Medacta’s commitment to develop solutions that are able to improve patient outcomes and healthcare system sustainability.”
The NextAR TKA is the first application of a new platform technology, which will be extended to hip, shoulder and spine procedures, designed and engineered with artificial intelligence and machine learning that make pre-operative CT-based planning and analysis efficient and precise.
The NextAR TKA Application is designed to improve efficiency and precision in total knee replacement and deliver personalized planning, with low upfront capital investment required by clinics and hospitals, as well as economic benefits to the healthcare system through OR efficiency and low cost per procedure. Medacta believes that this new platform will be an optimal solution particularly for U.S. ambulatory surgery centers (ASCs), which provide same-day surgical care.
The Augmented Reality glasses provided with the NextAR Platform allow the surgeon to visualize surgical actions and information in real-time, directly on the operative field. This improves the user experience and helps the surgeon remain focused on the patient at all times.
“With enhanced visualization via augmented reality, the NextAR Platform is an innovative and efficient tool for ultra-precise, patient-specific treatment,” said Dr Michael McAuliffe (MBBS FRACS), an orthopedic surgeon at St Andrew’s Ipswich Hospital in Queensland, Australia, and a member of the expert surgeon panel that Medacta collaborated with to develop the NextAR TKA Application. “With the NextAR TKA Application, we are able to track 3D soft tissue behavior during a surgery in real time. This functionality is an exciting advancement from traditional computer-assisted or robotic-assisted surgical systems that only track the relative movement of the knee bones, not the actual soft tissue.”
The NextAR TKA Application, with a precise reconstruction of the patient bone morphology, allows direct tracking of the collateral ligaments and a 3D analysis of soft tissue behavior throughout the whole range of motion during surgery, bringing patient-specific ligament balancing to the next level.
NextAR TS
In addition to advanced planning tools, Medacta has developed the NextAR TS, an infrared single-use tracking system that has the potential to vastly improve surgery efficiency while helping surgeons execute pre-op plans. Particularly in today’s environment, as surgeons and health systems look to recover surgeries that were deferred during the COVID-19 pandemic, efficiency in the operating room will become even more crucial than before. The NextAR Platform with its Single-Use Infrared Tracking System has the goal to meet these new levels of market demand.
The NextAR TKA works in conjunction with the Medacta GMK Sphere Medially Stabilized Knee implant, which has been proven to facilitate restoration of natural patient-specific kinematics.
Francesco Siccardi, CEO of Medacta, commented, “Patients and surgeons are attracted by improved outcomes and innovation, the NextAR Platform is one Medacta answer to the current race in orthopedic technology and I am very excited about this milestone achievement. Requiring a very limited investment in capital equipment, the NextAR Platform perfectly represents Medacta’s commitment to develop solutions that are able to improve patient outcomes and healthcare system sustainability.”