Nigel Syrotuck, Mechanical Engineer, StarFish Medical08.10.17
Nothing in medical devices is arguably more exciting than powered exoskeletons (check out this Wikipedia article listing all the movies in which they’ve been featured), so let’s take some time to examine them more closely and explore why they aren’t more prevalent in industry and medical treatment solutions.
Powered exoskeletons took their first (conceptual) step all the way back in 1890, and have been a work of both fact and fiction ever since. Backed by interest from military, medical, and industrial groups—3 of the largest markets in the world—attempts throughout the 20th century never quite pulled it off until a number of different groups demonstrated successful units by 2005. Powered exoskeletons have been available on the open market for a few years, but despite this they annually sell units in the hundreds—not the thousands or even millions you might expect, given that over 10 percent of Americans have mobility issues, 20 percent of whom are in a wheelchair.
How is it a technology that’s captivated human interest for over 125 years, accrued millions of dollars in funding, and been demonstrated successfully in a number of high-profile industries isn’t ubiquitous yet? Is it the calm before the storm? Or is the exoskeleton doomed to be a commercial, medical, and military failure? The obvious answer is they are too technically challenging, so let’s examine those challenges and see how they played out for two high-profile products.

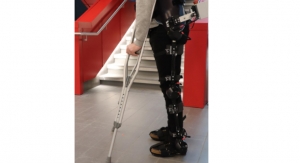
The ReWalker 6.0 is another example of a commercially available powered exoskeleton for lower limbs. Image courtesy of ReWalk Robotics.
Exoskeletons assist a person to use their own muscle power (via electro-muscular stimulation) or provide additional power with a powered exoskeleton, or both. To add power, it can use hydraulics, pneumatics, motors, or similar systems. They can be configured for biomimicry, where actuators contract wires just as muscles do to ligaments, or the system can be configured in a more traditionally mechanical approach, using torque drives and linkages. Either way, robust mechanical motions in robotics (spinning shafts, hinges, etc.) happen to be quite different from human ones. You probably don’t think about your elbow much, but it’s a rather challenging system to replicate with its hinge-like motion in one axis and twisting motion about another.
Understanding user dynamics is even more complex. What type of micro-movements do humans make? When and why do we make them? I have been fascinated by the TV show “The Expanse,” in which the characters use magnetic boots to walk around their spaceship in microgravity. These boots automatically disengage when a step begins, and then re-engage when a step is completing, allowing the user to feel like they’re walking on Earth. (It’s a sci-fi show, so rail guns and ion drives are par for the course.) However, differentiating a true step from a slight movement every time while also feeling natural to the user—even in harrowing life or death situations—is something this mechanical engineer can’t accept that easily.
There is an interesting quandary around assistive technologies. Hinge and motor exoskeleton systems have been around for years with some success, but they’re not perfect and tend to look hacked together. Alternatively, cutting edge structural materials are seemingly just around the corner. They offer compliant actuators, integrated sensors, and a better appearance and feeling, but aren’t proven to work. Which technologies receive backing could change the entire approach of the industry. If I were an investor, I’d have to carefully consider where to place my bets.
State of the Market
There hasn’t been an approved full-body device yet in the U.S. even though lower exoskeletons were first approved in 2014. At the moment, powered exoskeletons are likely in the “early adopter” market phase, meaning a number of interested customers are (hopefully) waiting to see if they are proven to be reliable, useful, and whether the price drops. A number of people who might be medically eligible view exoskeletons in a negative light as an attempt to fix people with disabilities, which narrows the market somewhat. For those interested, there are currently about 10 different units for sale to the public averaging about $70,000 each, a prohibitive cost for many. Despite these factors, the market is expected to be worth $3.3B by 2025 with a number of parties touting newer, better versions in the next few years (take the U.S. military’s 2018 goal of the TALOS, for example).
The automobile is an interesting comparison of a large, paradigm shifting mechanical technology with a set of human factors. The “modern” car was first produced in the late 1800’s. Decades later, Henry Ford drove the Model T’s famous release in 1908 at the price of $825 ($18,000 in 2017). Automobiles did not accelerate in sales for a number of years, eventually overtaking horse carriages a decade later after their price had dropped by 40 percent (costing around $500 or $7,000 in 2017). Following that model, the price of exoskeletons must drop 40 percent to invigorate their sales dramatically. However, that leaves customers and healthcare providers with a $42,000 price tag, still a hard sell compared to a $300 wheelchair. Of course, the model T was relatively simple compared to today’s cars, which now average over $30,000 each, and they didn’t hold to strict regulatory standards like medical devices.

"Alexa, let's walk to the kitchen!" Bionik Labs' ARKE exoskeleton was recently outfitted with Amazon Echo. Image courtesy of Bionik Laboratories.
Similar to powered exoskeletons, da Vinci surgical robots are a complex electro-mechanical system with integral human factors challenges. They are about ten years ahead of exoskeletons, having been approved by the FDA in 2000. Like exoskeletons, the future of da Vinci robots isn’t crystal clear, but they are progressing in the market well with at least 3,803 installed worldwide. This is a critical time in the business; performance flaws in the installed base are under scrutiny and a net positive cash flow is critical. It’s likely powered exoskeletons will also come under closer scrutiny in the next ten years once they become more common, perhaps explaining (along with the technical challenges) why there doesn’t seem to be a group willing to make the shift into mass production until more kinks have been ironed out.
There are a number of reasons that powered exoskeletons aren’t ubiquitous yet. Powered exoskeletons are hard to make and even harder to make well. It is pure science fiction to expect them to feel natural to the user any time soon. They’re expensive to build, expensive to buy, and likely unreliable in the long term. Even more challenging is a huge breakthrough (or two) in materials science might make all progress on current hardware-based suits obsolete, questioning the wisdom of investing now. Fewer than 10 groups worldwide are selling powered exoskeletons on the open market, and even those are only for lower extremity support. Regardless, they have the potential to provide a lot of benefits to people across the world. The lack of sales or an exploding market share can be a disillusion to some, but it took the modern car about 35 years to go from concept to common, and they certainly were not cheap. It seems like powered exoskeletons still have another decade or so before we should start to worry that an affordable, accessible, functional exoskeleton may never happen.
What do you think? Will powered exoskeletons make a grand step into the market anytime soon?
Nigel Syrotuck is a mechanical engineer for Victoria, BC, Canada-based medical device design, development, and contract manufacturing firm StarFish Medical.
Powered exoskeletons took their first (conceptual) step all the way back in 1890, and have been a work of both fact and fiction ever since. Backed by interest from military, medical, and industrial groups—3 of the largest markets in the world—attempts throughout the 20th century never quite pulled it off until a number of different groups demonstrated successful units by 2005. Powered exoskeletons have been available on the open market for a few years, but despite this they annually sell units in the hundreds—not the thousands or even millions you might expect, given that over 10 percent of Americans have mobility issues, 20 percent of whom are in a wheelchair.
How is it a technology that’s captivated human interest for over 125 years, accrued millions of dollars in funding, and been demonstrated successfully in a number of high-profile industries isn’t ubiquitous yet? Is it the calm before the storm? Or is the exoskeleton doomed to be a commercial, medical, and military failure? The obvious answer is they are too technically challenging, so let’s examine those challenges and see how they played out for two high-profile products.

The ReWalker 6.0 is another example of a commercially available powered exoskeleton for lower limbs. Image courtesy of ReWalk Robotics.
Understanding user dynamics is even more complex. What type of micro-movements do humans make? When and why do we make them? I have been fascinated by the TV show “The Expanse,” in which the characters use magnetic boots to walk around their spaceship in microgravity. These boots automatically disengage when a step begins, and then re-engage when a step is completing, allowing the user to feel like they’re walking on Earth. (It’s a sci-fi show, so rail guns and ion drives are par for the course.) However, differentiating a true step from a slight movement every time while also feeling natural to the user—even in harrowing life or death situations—is something this mechanical engineer can’t accept that easily.
There is an interesting quandary around assistive technologies. Hinge and motor exoskeleton systems have been around for years with some success, but they’re not perfect and tend to look hacked together. Alternatively, cutting edge structural materials are seemingly just around the corner. They offer compliant actuators, integrated sensors, and a better appearance and feeling, but aren’t proven to work. Which technologies receive backing could change the entire approach of the industry. If I were an investor, I’d have to carefully consider where to place my bets.
State of the Market
There hasn’t been an approved full-body device yet in the U.S. even though lower exoskeletons were first approved in 2014. At the moment, powered exoskeletons are likely in the “early adopter” market phase, meaning a number of interested customers are (hopefully) waiting to see if they are proven to be reliable, useful, and whether the price drops. A number of people who might be medically eligible view exoskeletons in a negative light as an attempt to fix people with disabilities, which narrows the market somewhat. For those interested, there are currently about 10 different units for sale to the public averaging about $70,000 each, a prohibitive cost for many. Despite these factors, the market is expected to be worth $3.3B by 2025 with a number of parties touting newer, better versions in the next few years (take the U.S. military’s 2018 goal of the TALOS, for example).
The automobile is an interesting comparison of a large, paradigm shifting mechanical technology with a set of human factors. The “modern” car was first produced in the late 1800’s. Decades later, Henry Ford drove the Model T’s famous release in 1908 at the price of $825 ($18,000 in 2017). Automobiles did not accelerate in sales for a number of years, eventually overtaking horse carriages a decade later after their price had dropped by 40 percent (costing around $500 or $7,000 in 2017). Following that model, the price of exoskeletons must drop 40 percent to invigorate their sales dramatically. However, that leaves customers and healthcare providers with a $42,000 price tag, still a hard sell compared to a $300 wheelchair. Of course, the model T was relatively simple compared to today’s cars, which now average over $30,000 each, and they didn’t hold to strict regulatory standards like medical devices.

"Alexa, let's walk to the kitchen!" Bionik Labs' ARKE exoskeleton was recently outfitted with Amazon Echo. Image courtesy of Bionik Laboratories.
Similar to powered exoskeletons, da Vinci surgical robots are a complex electro-mechanical system with integral human factors challenges. They are about ten years ahead of exoskeletons, having been approved by the FDA in 2000. Like exoskeletons, the future of da Vinci robots isn’t crystal clear, but they are progressing in the market well with at least 3,803 installed worldwide. This is a critical time in the business; performance flaws in the installed base are under scrutiny and a net positive cash flow is critical. It’s likely powered exoskeletons will also come under closer scrutiny in the next ten years once they become more common, perhaps explaining (along with the technical challenges) why there doesn’t seem to be a group willing to make the shift into mass production until more kinks have been ironed out.
There are a number of reasons that powered exoskeletons aren’t ubiquitous yet. Powered exoskeletons are hard to make and even harder to make well. It is pure science fiction to expect them to feel natural to the user any time soon. They’re expensive to build, expensive to buy, and likely unreliable in the long term. Even more challenging is a huge breakthrough (or two) in materials science might make all progress on current hardware-based suits obsolete, questioning the wisdom of investing now. Fewer than 10 groups worldwide are selling powered exoskeletons on the open market, and even those are only for lower extremity support. Regardless, they have the potential to provide a lot of benefits to people across the world. The lack of sales or an exploding market share can be a disillusion to some, but it took the modern car about 35 years to go from concept to common, and they certainly were not cheap. It seems like powered exoskeletons still have another decade or so before we should start to worry that an affordable, accessible, functional exoskeleton may never happen.
What do you think? Will powered exoskeletons make a grand step into the market anytime soon?
Nigel Syrotuck is a mechanical engineer for Victoria, BC, Canada-based medical device design, development, and contract manufacturing firm StarFish Medical.