Ben White, Engineering Manager, Business Development, Millstone Medical Outsourcing02.13.19
Time to market is critical to success for OEMs when it comes to launching new product lines and devices. Yet, despite the pressure and desire to speed time to market, an OEM can never lose sight of the top priorities of product quality and patient safety. The risk is otherwise continual, and unanticipated delays can impact product launches; or, in worst-case scenarios, new technologies never reach the market at all. With the rise of robust pre-validated packaging and universal packaging solutions, preserving quality while cutting time to market by up to 50 percent is possible. This article examines the rise of pre-validated and universal packaging solutions, the promise of compressed product launch timelines, and the risk mitigation these options offer.
Increased Scrutiny for a Growing Market
The medical implants market is growing rapidly, projected to garner $116 billion by 2022.1 Orthopedic implants represent the largest growth sector of this market, as an increasing number of patients ages 55+ worldwide opt for surgeries that improve and extend quality of life. An increase in the aging population of the United States as well as increased adoption in markets around the world, such as Asia, are contributing to worldwide growth in the orthopedic implant market. In response to this growth as well as to technological advancements that fuel innovation, new product development proliferates, from the creation of patient-specific implants to the application of 3D technology and sculptural computer-aided design.
As the market has grown, scrutiny and regulatory oversight has increased, which compounds the complexity in process and package validation. The FDA has established classification for nearly 2,000 medical devices, sorting them into three regulatory classes based on the level of control considered necessary to ensure device safety and effectiveness in patients. At the same time, scrutiny of the impact of devices on patients and patient safety has increased, with the FDA focusing on objective evidence from OEMs on each portion of the validation process to be in compliance. In addition, significant revisions to ISO 11607 have been made for the first time in more than a decade, necessitating an increased focus on materials selection, design, process, and quality controls. For a final layer of complexity in an already complex landscape, individual OEMs may approach package validation differently, based on how they individually interpret ISO regulations.
As a result, packaging design and validation is a multifaceted process that can take significant time and planning, increasing time to market for OEMs. In some cases, the time required for package design and validation can be 12 to 18 months, or even longer. A new package design process offers no guarantees; just because an OEM is spending valuable time and resources designing a package doesn’t mean that package is in fact going to pass validation.
A Brief Overview of Package Validation
Package validation is the process of creating and ensuring the integrity of a sterile package for an implant’s transfer from manufacturer to patient. After all, implants must enter and exist within the human body without negative patient implications. To safeguard patient safety, package validations ensure implants withstand transportation to the operating room with effective aseptic transfer into the surgical suite and into patients’ bodies. Package validation is also mandatory for compliance with regulatory oversight and global standards that govern packaging, such as the ISO 11607 standard (Parts 1 and 2).
The ISO 11607 standard defines three key components of a validated packaging system:
A standard validation process follows an installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). To illustrate, consider the sealing process, which is necessary to create the sterile barrier. The sealing IQ ensures the sealing equipment is installed and operates properly. After a thorough design of experiment (DOE) to determine viable input parameters, an OQ is conducted to test the sealing process at certain challenge conditions. The sealing PQ tests nominal parameters for effective, reproducible results with induced variability (e.g., multiple operators, product lots, shifts, etc.).
Challenging a sealing process is based on a variety of inputs and controls, such as a selection of representable product, sealing equipment controls (e.g., temperature, pressure, dwell time, etc.). In short, all qualifications are executed to provide sufficient confidence and evidence that patient risk is mitigated.
Best Practice: A Holistic Approach to Product and Packaging Design
With so many layers of complexity and so much pressure on reaching the market quickly and safely, OEMs face an uphill climb when it comes to design and validation. To mitigate risk, prioritize patient safety, and speed time to market, it’s essential to consider product and package design as two parts of a whole.
In an ideal scenario, an OEM takes a holistic approach, simultaneously developing the device while determining how to design its package and handle the validation process successfully. Considerations of the stresses of distribution as well as the aseptic transfer within the surgical suite are present throughout the design process.
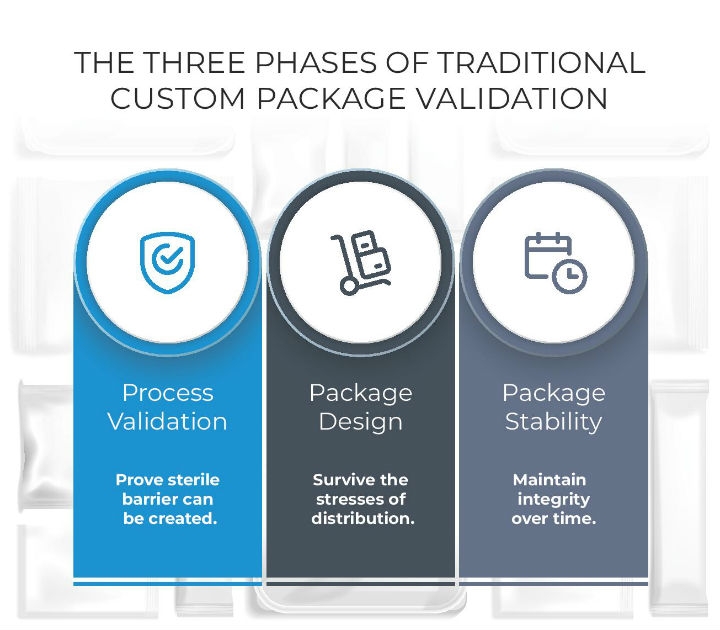
Figure 1
Traditional custom package validation falls into three phases (Figure 1):
Cutting Time to Market
Traditionally, accelerating time to market has hinged on preparation and process organization, even in the best cases when product and package design are considered holistically. OEMs and sterile package manufacturers must choose a sterile barrier system, define a cleaning and sterilization process, and undergo distribution and aging testing.
Depending on the approach to distribution and accelerated aging testing an OEM takes, the validation timeframe can be 26 weeks or more. The rise of robust solutions for pre-validated and universal packaging configurations can eliminate up to 13 weeks from a typical validation timeline, cutting time to market by 50 percent (Figure 2).
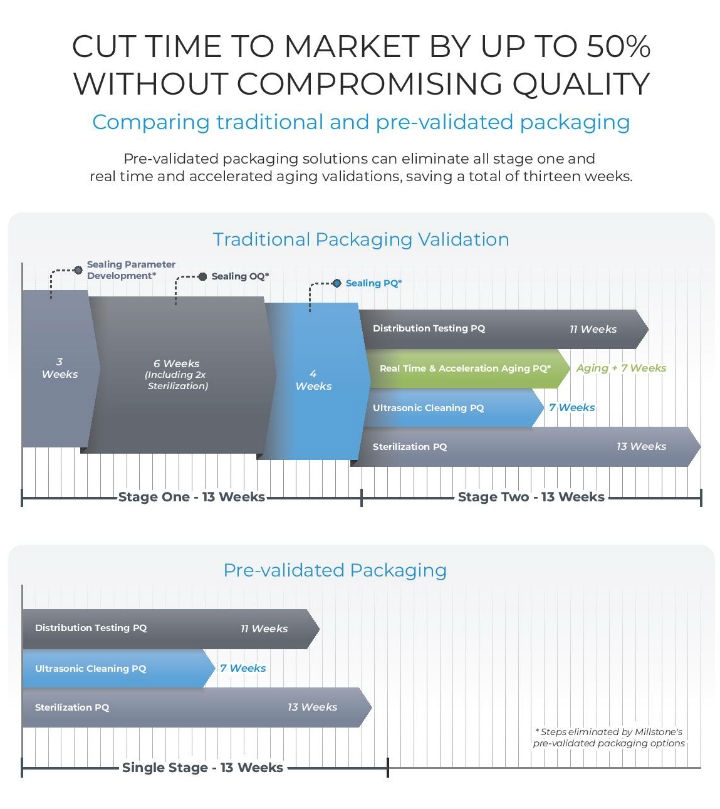
Figure 2
Six Key Validation Benefits to OEMs
The rise of pre-validated and universal package configurations introduces an array of benefits and efficiencies for OEMs when it comes to the validation process.
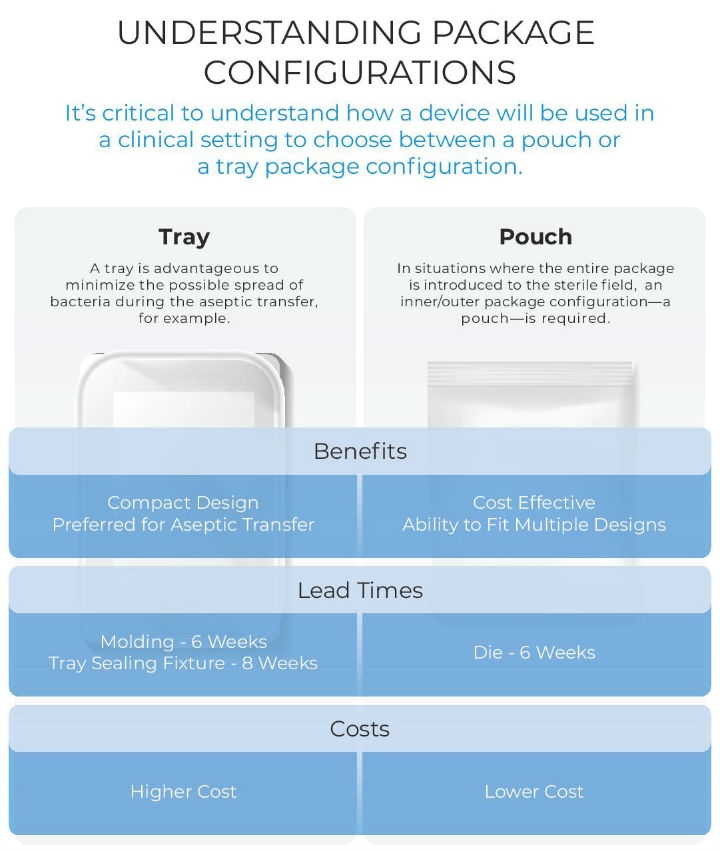
Figure 3
A Solution for Remediated Packaging
In some cases, an OEM becomes mired in the package design and validation process. This can happen as a result of quality or design gaps, or because of an in-depth FDA audit that flags issues with compliance. It can also happen when package validation keeps failing at a specific point in the process, for example in accelerated aging, when the OEM introduces parameters that a package cannot withstand in an effort to compress accelerated aging timelines and get to market more quickly.
In these cases, pre-validated packaging, especially a solution offered by an industry expert, can prove beneficial. Package design and validation is outsourced, and the vendor or partner can remediate the process with pre-validated packaging that gets the product into compliance—and to market—more quickly.
Considerations and Concerns
The use of pre-validated packaging or universal packaging solutions, of course, doesn’t eliminate the necessity for ongoing operational qualification and performance qualification, essential components of compliance with ISO 11607. Ensuring all validation data is maintained in an OEM’s design history file is essential because, of course, a manufacturer is ultimately responsible when it comes to compliance, an audit, a recall, or any other adverse event.
Why Outsource Package Validation?
For OEMs, balancing time to market with product safety can feel like a tug of war. The complexities of package design and validation and the rigors of maintaining ISO standards in the process can drive up headcount, operating, and fixed costs.
Outsourcing to an industry partner with market expertise in packaging design and validation allows OEMs to focus where they excel—on creating great products that improve patients’ lives. Pre-validated packaging can enable OEMs to safely deliver products to market faster and increase OEMs’ confidence in the quality of the finished goods. At the same time, outsourcing can manage the risk of validation and ensure there are no regulatory constraints or delays. It can also alleviate some of the challenges a manufacturer faces in proving it maintains a validated state. On a broader scale, outsourcing also frees resources and talent to focus on an OEM’s core competencies of product design, marketing, and sales, fueling growth in the customer base and the business overall.
There’s an additional consideration when it comes to the expertise made available to an OEM through outsourcing. Access to the latest perspective and industry best practices on the interpretation of ISO standards, the current state of the regulatory landscape, and the latest FDA activities gives OEMs invaluable insight on strategy for current as well as future products.
Conclusion
Robust pre-validated and universal packaging configuration solutions offer a great deal of promise for manufacturers in balancing patient safety with time-to-market considerations. In reducing risk, cost, and unnecessary delay while introducing cost efficiencies, pre-validated packaging can help OEMs better navigate the challenge of taking an optimal and holistic approach to product and packaging design.
Reference
1 http://bit.ly/odt190140
Ben White is the engineering manager for business development at Millstone Medical Outsourcing. He has been with the company for five years in roles in quality and validation engineering. Through partnerships with top orthopedic companies, White’s team stands at the forefront of industry regulations regarding sterile packaging, end of line cleaning, and sterilization.
Increased Scrutiny for a Growing Market
The medical implants market is growing rapidly, projected to garner $116 billion by 2022.1 Orthopedic implants represent the largest growth sector of this market, as an increasing number of patients ages 55+ worldwide opt for surgeries that improve and extend quality of life. An increase in the aging population of the United States as well as increased adoption in markets around the world, such as Asia, are contributing to worldwide growth in the orthopedic implant market. In response to this growth as well as to technological advancements that fuel innovation, new product development proliferates, from the creation of patient-specific implants to the application of 3D technology and sculptural computer-aided design.
As the market has grown, scrutiny and regulatory oversight has increased, which compounds the complexity in process and package validation. The FDA has established classification for nearly 2,000 medical devices, sorting them into three regulatory classes based on the level of control considered necessary to ensure device safety and effectiveness in patients. At the same time, scrutiny of the impact of devices on patients and patient safety has increased, with the FDA focusing on objective evidence from OEMs on each portion of the validation process to be in compliance. In addition, significant revisions to ISO 11607 have been made for the first time in more than a decade, necessitating an increased focus on materials selection, design, process, and quality controls. For a final layer of complexity in an already complex landscape, individual OEMs may approach package validation differently, based on how they individually interpret ISO regulations.
As a result, packaging design and validation is a multifaceted process that can take significant time and planning, increasing time to market for OEMs. In some cases, the time required for package design and validation can be 12 to 18 months, or even longer. A new package design process offers no guarantees; just because an OEM is spending valuable time and resources designing a package doesn’t mean that package is in fact going to pass validation.
A Brief Overview of Package Validation
Package validation is the process of creating and ensuring the integrity of a sterile package for an implant’s transfer from manufacturer to patient. After all, implants must enter and exist within the human body without negative patient implications. To safeguard patient safety, package validations ensure implants withstand transportation to the operating room with effective aseptic transfer into the surgical suite and into patients’ bodies. Package validation is also mandatory for compliance with regulatory oversight and global standards that govern packaging, such as the ISO 11607 standard (Parts 1 and 2).
The ISO 11607 standard defines three key components of a validated packaging system:
- Package system design and development, including materials selection, design, and packaging system design qualification testing
- Qualification, validation, and proof of stability of sterile barrier system (SBS) materials and seals
- SBS sealing process validation
A standard validation process follows an installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). To illustrate, consider the sealing process, which is necessary to create the sterile barrier. The sealing IQ ensures the sealing equipment is installed and operates properly. After a thorough design of experiment (DOE) to determine viable input parameters, an OQ is conducted to test the sealing process at certain challenge conditions. The sealing PQ tests nominal parameters for effective, reproducible results with induced variability (e.g., multiple operators, product lots, shifts, etc.).
Challenging a sealing process is based on a variety of inputs and controls, such as a selection of representable product, sealing equipment controls (e.g., temperature, pressure, dwell time, etc.). In short, all qualifications are executed to provide sufficient confidence and evidence that patient risk is mitigated.
Best Practice: A Holistic Approach to Product and Packaging Design
With so many layers of complexity and so much pressure on reaching the market quickly and safely, OEMs face an uphill climb when it comes to design and validation. To mitigate risk, prioritize patient safety, and speed time to market, it’s essential to consider product and package design as two parts of a whole.
In an ideal scenario, an OEM takes a holistic approach, simultaneously developing the device while determining how to design its package and handle the validation process successfully. Considerations of the stresses of distribution as well as the aseptic transfer within the surgical suite are present throughout the design process.

Figure 1
Traditional custom package validation falls into three phases (Figure 1):
- Process validation, which proves that a sterile barrier can be created.
- Package design, which is event-related and concerns whether or not the design enables the device to survive distribution with an integral sterile barrier from distribution through the arrival in the operating room.
- Package stability, which is time-related and entails proving the packaging maintains its integrity for the shelf life determined by the OEM. Proof of this can be addressed through accelerated aging techniques—if a product will have a shelf life of five years, it’s not necessary for the OEM to wait the full five years. Instead, accelerated aging can simulate the aging process and provide the necessary proof.
Cutting Time to Market
Traditionally, accelerating time to market has hinged on preparation and process organization, even in the best cases when product and package design are considered holistically. OEMs and sterile package manufacturers must choose a sterile barrier system, define a cleaning and sterilization process, and undergo distribution and aging testing.
Depending on the approach to distribution and accelerated aging testing an OEM takes, the validation timeframe can be 26 weeks or more. The rise of robust solutions for pre-validated and universal packaging configurations can eliminate up to 13 weeks from a typical validation timeline, cutting time to market by 50 percent (Figure 2).

Figure 2
Six Key Validation Benefits to OEMs
The rise of pre-validated and universal package configurations introduces an array of benefits and efficiencies for OEMs when it comes to the validation process.
- Reduced risk: First and foremost, pre-validated packaging greatly reduces risk in the validation process when compared to custom validated packaging. As noted earlier, package design comes with no guarantee when it comes to validation, and there are many points in the process where a package can fail an associated validation. Examples include pain points on a device and even the parameters utilized in the accelerated aging process, such as testing with aggressively high temperature ranges. Because pre-validated packaging is, by its very nature, already validated, an OEM has the assurance a sterile barrier has been created and it can be maintained; and the package has withstood the rigors of distribution and aging testing. Key knowledge of the factors involved in successfully completing distribution and accelerated aging testing also goes into the design of certain pre-validated and universal packaging solutions. For instance, understanding the change of pressure that a device will experience in airplane transit, or the shock stress of transportation by a truck, can be incorporated into pre-validated designs, heading off the potential for these distribution stresses to delay time to market in custom packaging design. Similarly, thorough understanding of optimal temperature and humidity ranges for accelerated aging testing can avoid unnecessary delays in the validation process.
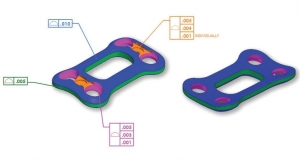
- Flexible package design: Certain solutions offer flexible package designs with a validated sterile barrier or sealing flange and can be used for a variety of spine and orthopedic designs. Whether an OEM chooses a pouch or tray design (Figure 3), manipulation of an inner insert or the geometry within the sterile barrier can accommodate a wide range of product SKUs within one single type of pre-validated sterile package. Flexibility in package design can be especially beneficial to small or medium-sized OEMs that might not have a large product engineering and design staff and where resources may be spread more thinly. For these manufacturers, a pre-validated option with inserts sized specifically to accommodate particular products of different sizes in a product line significantly speeds time to market while saving costs. Products can be packaged in trays or pouches within an outer package that has a pre-validated sterile barrier. It’s critical to understand how a device will be used in a clinical setting to choose between a pouch or tray package configuration. A tray is advantageous to minimize the possible spread of bacteria during the aseptic transfer, for example. In situations where the entire package is introduced to the sterile field, rather than just its contents, an inner/outer package configuration is required.
- Flexibility in sterilization method: Pre-validated packaging also offers flexibility in the method used for sterilization, accommodating gamma, ethylene oxide (EO), or E-beam sterilization pending specific product needs. For instance, sterilization by EO requires a semi-permeable sterile barrier, which allows the sterilization gas to enter the packaging. Pre-validated solutions that factor in such detail can stave off significant delays in the validation process.
- Resource efficiencies: Pre-validated packaging introduces several areas for resource efficiencies. Approaching the process with a universal packaging design allows for use by multiple product families independent of geometry and weight. In addition, a pre-validated or universal solution can mean fewer materials are needed, simplifying the supply chain and introducing greater certainty when it comes to materials use. A consolidated package design can also reduce the number of decisions an OEM or its staff faces when it comes to packaging, material, and the validation process.
- Reduced costs: Pre-validated packaging reduces upfront costs while managing risk. If, for instance, an OEM introduces additional SKUs with the same materials and parameters as devices that previously passed validation, the same type of pre-validated packaging solution can be used. As a result, the necessity for repetitive testing, which adds time and cost to the validation process, can be reduced or eliminated.
- Mistake-proofing: Pre-validated packaging can reduce or eliminate the potential for human error, which can in turn cut risk and improve quality assurance. For instance, under pressure to get to market, custom package design can sometimes result in the design of multiple trays and lids, which can be put together in incorrect configurations. Certain pre-validated or universal packaging solutions have been designed so that there is only one way to assemble the final package, mistake-proofing the process and introducing operator efficiency while also reducing risk.

Figure 3
A Solution for Remediated Packaging
In some cases, an OEM becomes mired in the package design and validation process. This can happen as a result of quality or design gaps, or because of an in-depth FDA audit that flags issues with compliance. It can also happen when package validation keeps failing at a specific point in the process, for example in accelerated aging, when the OEM introduces parameters that a package cannot withstand in an effort to compress accelerated aging timelines and get to market more quickly.
In these cases, pre-validated packaging, especially a solution offered by an industry expert, can prove beneficial. Package design and validation is outsourced, and the vendor or partner can remediate the process with pre-validated packaging that gets the product into compliance—and to market—more quickly.
Considerations and Concerns
The use of pre-validated packaging or universal packaging solutions, of course, doesn’t eliminate the necessity for ongoing operational qualification and performance qualification, essential components of compliance with ISO 11607. Ensuring all validation data is maintained in an OEM’s design history file is essential because, of course, a manufacturer is ultimately responsible when it comes to compliance, an audit, a recall, or any other adverse event.
Why Outsource Package Validation?
For OEMs, balancing time to market with product safety can feel like a tug of war. The complexities of package design and validation and the rigors of maintaining ISO standards in the process can drive up headcount, operating, and fixed costs.
Outsourcing to an industry partner with market expertise in packaging design and validation allows OEMs to focus where they excel—on creating great products that improve patients’ lives. Pre-validated packaging can enable OEMs to safely deliver products to market faster and increase OEMs’ confidence in the quality of the finished goods. At the same time, outsourcing can manage the risk of validation and ensure there are no regulatory constraints or delays. It can also alleviate some of the challenges a manufacturer faces in proving it maintains a validated state. On a broader scale, outsourcing also frees resources and talent to focus on an OEM’s core competencies of product design, marketing, and sales, fueling growth in the customer base and the business overall.
There’s an additional consideration when it comes to the expertise made available to an OEM through outsourcing. Access to the latest perspective and industry best practices on the interpretation of ISO standards, the current state of the regulatory landscape, and the latest FDA activities gives OEMs invaluable insight on strategy for current as well as future products.
Conclusion
Robust pre-validated and universal packaging configuration solutions offer a great deal of promise for manufacturers in balancing patient safety with time-to-market considerations. In reducing risk, cost, and unnecessary delay while introducing cost efficiencies, pre-validated packaging can help OEMs better navigate the challenge of taking an optimal and holistic approach to product and packaging design.
Reference
1 http://bit.ly/odt190140
Ben White is the engineering manager for business development at Millstone Medical Outsourcing. He has been with the company for five years in roles in quality and validation engineering. Through partnerships with top orthopedic companies, White’s team stands at the forefront of industry regulations regarding sterile packaging, end of line cleaning, and sterilization.