Sean Fenske, Editor-in-Chief02.18.20
The healthcare industry is historically slow to adopt new technologies that could create opportunities for greater efficiencies. Some of that is woven into the fabric of the industry; the delivery of care requires utmost assurance of quality while also being under the umbrella of FDA oversight. It makes for an environment that wants solutions to be fully vetted before they are implemented.
Fortunately, Vic Nunes, CEO and founder of QMed Innovations and principal at On Point Business Advisors (a consulting firm serving the medical device industry), is quite familiar with this dynamic. He has held executive positions at DePuy Synthes and is currently a member of the board of directors at N2 Biomedical. During his tenure at DePuy, he witnessed first-hand the challenges involved with surgical kits the company would deploy to support its customers.
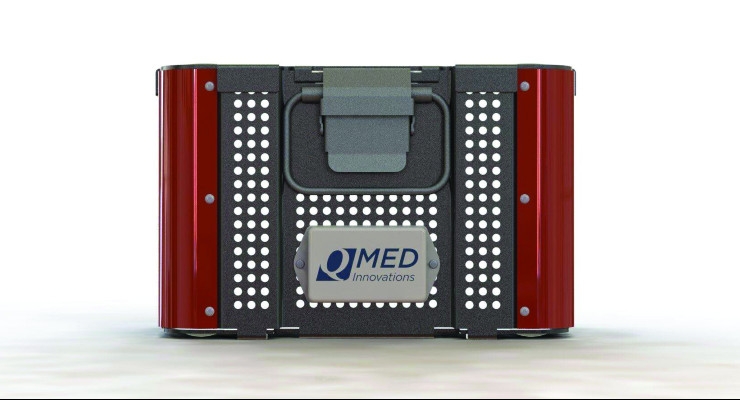
Perhaps as a result of that experience, Nunes formed QMed Innovations, which developed Quest. Quest offers tracking and status information of a firm’s kits in real time. At a glance, a manager at an orthopedic company can see where kits are located, whether they have been decontaminated, how much they’ve been used since being recalibrated, and more.
Nunes took time to speak with ODT about his new system, why it’s necessary, what its benefits are, and what gains can be realized from its implementation.
Sean Fenske: What is Quest and why should orthopedic device manufacturers be aware of it?
Victor Nunes: I spent over 25 years in the orthopedic industry as a senior executive, managing supply chain and inventory across the globe. Each year during budget review, the enormous spend on new kit inventory we were deploying was evident. No one was really too concerned with inventory levels 15 years ago, because margins were high and we could get price growth from year to year.
The healthcare economic environment has changed significantly since then; the industry can no longer afford to continue to deploy inventory without real visibility as to how efficient inventory is being used in the field. Consignment inventory is great as long as it gets used, but that’s not necessarily the case in our industry.
Quest is the first real solution in the marketplace where an orthopedic company has total visibility to pertinent daily data that monitors the whereabouts of a company’s kits, provides key information on usage or inventory turns, monitors instrument lifecycle, and offers many other reports to help maximize inventory efficiency.
Fenske: What actually comprises the technology?
Nunes: Quest is a combination hardware device and custom software. We have designed this solution to be a system, but to also function independently and connect with any software the customer may already have in place.
Fenske: How does Quest track kits? What connectivity solution is used?
Nunes: First, we wanted to develop a technology that would perform all of the tasks required in an automated fashion and be independent from any human or machine inputs. That was key to our business model. Quest does not require any sort of infrastructure, scanners, or manual interaction to track and report data. Quest is an IoT device that operates on a low-power wide-area network (LPWAN). This means our technology operates at very low frequencies and is capable of transmitting through structures where a cell transmission may not. It also has a GPS device incorporated but position location is acquired with other proprietary technology beyond GPS.
Fenske: What other information about the kits can be captured?
Nunes: Quest was developed to give an orthopedic company accurate visibility on the location of its kits on a daily basis. There are also a number of outputs we capture with other sensors, such as number of sterilization cycles. From there, we can determine the number of surgeries and track inventory turns.
One area hardly ever discussed is the lack of an ability to ensure all instrumentation is calibrated or in perfect condition for the next surgery. Patient risk and safety is at the core of this issue. If the industry can’t tell you where and how often a kit has been used, how can anyone guarantee the kit is ready for the next surgery? With Quest we know everything about the kit, where it is, where it was at a specific date, how often it has been in surgery. It keeps track of the number of surgeries, has the ability to monitor instruments’ lifecycles, and can issue an alert when the kit needs to be returned for inspection and calibration. We also offer a kit reservation system that allows employees to schedule kits in advance.
Imagine being able to collect key data for your field inventory, have the ability to redeploy inventory across territories, make better decisions on what should be consigned or what should be in a loaner pool, make more accurate decisions on how much new inventory needs to be built, and, perhaps most important, bring accountability and management to very expensive assets.
Fenske: You mentioned no interaction is required, whether human or machine input. Does that include the medical professional using the kits within their facility?
Nunes: That’s the beauty of this system. None whatsoever. Once Quest is installed on the kit, it’s live, capturing data, and reporting. The only thing we need to do is conduct a short training on the software. There’s no additional infrastructure requirements, special receivers, or receptors needed. Quest has a proprietary logarithm that not only tells a user where it is located, but it also captures the hospital or customer warehouse location by name.
Fenske: What is the real takeaway for the orthopedic device manufacturer from a business perspective?
Nunes: We often talk about what Quest offers as a new technology. It’s like the difference between being totally blind and having 20/20 vision. I know how much this industry is spending annually on new kits, which, by the way, is the same as the industry’s revenue growth. Inventory turns are deplorable and the industry is behind the times on technology. This industry can’t afford to manage inventory in this way any longer.
Like any other industry, the moment you have real-time, accurate data on a daily basis, the entire business model can be improved because you’re not looking at a snapshot in time. Quest offers more information and capabilities to allow orthopedic leadership to re-invent the entire supply chain and redesign how they interact with customers.
Fenske: With a kit that’s already developed and being deployed by a company, will design adjustments be required before Quest can be used?
Nunes: As we work with each customer, our primary focus is new product launches or new inventory being deployed to the field. The case and tray will be designed with Quest in mind, but we will also have a bracket to secure Quest to existing inventory if the customer chooses to deploy it that way.
QMed is not in the business of selling Quest; we are in the business of solving customer’s inventory management problems with Quest. Each customer will be different and each customer will have their own specific requirements. We are set up to work with each customer on their specific needs. We do not intend to use Quest and the software as a one-size-fits-all approach.
Fenske: So ideally, Quest would be designed into a new product so as to better facilitate the solution?
Nunes: Absolutely. Quest is only the start; we are well aware there are plenty of opportunities for a complete “smart kit” with specific instrument data, eventually acquiring data that tracks instrument usage for optimum kit design or, more specifically, surgeon kit preference. There are also logistics opportunities to help alert when a kit is out of decontamination at a hospital so it can be picked up. Further, there are opportunities with automating the kit reservation system to the hospital surgery scheduling system. Our mission is to provide supply chain optimization through connected technology.
Fenske: What are the next steps for QMed Innovations and the Quest system?
Nunes: In the very short term, educate the industry on our technology. We have developed a very simple business model that allows complete visibility each step of the way of the sales process. In other words, we will develop a complete ROI with the customer, then develop a customer-specific pilot program at a very affordable cost before any significant investment is made. The ROI is simple, but it will help customers realize the added benefits Quest brings.
We are aware of the skepticism within the industry regarding technology and its abilities to address inventory management. As I mentioned, I come from the industry and I was part of leadership teams frustrated with the lack of complete systems to help us better manage our assets. I get it.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Nunes: The way the orthopedic industry conducts business and its relationship with customers has not changed and everyone’s model is the virtually the same. This includes the management of inventory (i.e., consignment, trunk stock, or loaner); interaction with customers (whether distributor or dedicated sales force); the role of the sales rep in the field; how much time they spend on non-selling activities such as logistics and chasing down kits; and management of the instrument lifecycle.
Quest and the data it provides opens the door to change the paradigm of how each orthopedic company will do business and its relationship with the customer. Data and technology is lacking in this industry; without it, business is stagnant. We’re trying to do our part to introduce this type of technology to the industry to cause a paradigm shift in inventory management.
To learn more about Quest and QMed Innovations, visit the company at www.qmedinnovations.com.
Fortunately, Vic Nunes, CEO and founder of QMed Innovations and principal at On Point Business Advisors (a consulting firm serving the medical device industry), is quite familiar with this dynamic. He has held executive positions at DePuy Synthes and is currently a member of the board of directors at N2 Biomedical. During his tenure at DePuy, he witnessed first-hand the challenges involved with surgical kits the company would deploy to support its customers.
Perhaps as a result of that experience, Nunes formed QMed Innovations, which developed Quest. Quest offers tracking and status information of a firm’s kits in real time. At a glance, a manager at an orthopedic company can see where kits are located, whether they have been decontaminated, how much they’ve been used since being recalibrated, and more.
Nunes took time to speak with ODT about his new system, why it’s necessary, what its benefits are, and what gains can be realized from its implementation.
Sean Fenske: What is Quest and why should orthopedic device manufacturers be aware of it?
Victor Nunes: I spent over 25 years in the orthopedic industry as a senior executive, managing supply chain and inventory across the globe. Each year during budget review, the enormous spend on new kit inventory we were deploying was evident. No one was really too concerned with inventory levels 15 years ago, because margins were high and we could get price growth from year to year.
The healthcare economic environment has changed significantly since then; the industry can no longer afford to continue to deploy inventory without real visibility as to how efficient inventory is being used in the field. Consignment inventory is great as long as it gets used, but that’s not necessarily the case in our industry.
Quest is the first real solution in the marketplace where an orthopedic company has total visibility to pertinent daily data that monitors the whereabouts of a company’s kits, provides key information on usage or inventory turns, monitors instrument lifecycle, and offers many other reports to help maximize inventory efficiency.
Fenske: What actually comprises the technology?
Nunes: Quest is a combination hardware device and custom software. We have designed this solution to be a system, but to also function independently and connect with any software the customer may already have in place.
Fenske: How does Quest track kits? What connectivity solution is used?
Nunes: First, we wanted to develop a technology that would perform all of the tasks required in an automated fashion and be independent from any human or machine inputs. That was key to our business model. Quest does not require any sort of infrastructure, scanners, or manual interaction to track and report data. Quest is an IoT device that operates on a low-power wide-area network (LPWAN). This means our technology operates at very low frequencies and is capable of transmitting through structures where a cell transmission may not. It also has a GPS device incorporated but position location is acquired with other proprietary technology beyond GPS.
Fenske: What other information about the kits can be captured?
Nunes: Quest was developed to give an orthopedic company accurate visibility on the location of its kits on a daily basis. There are also a number of outputs we capture with other sensors, such as number of sterilization cycles. From there, we can determine the number of surgeries and track inventory turns.
One area hardly ever discussed is the lack of an ability to ensure all instrumentation is calibrated or in perfect condition for the next surgery. Patient risk and safety is at the core of this issue. If the industry can’t tell you where and how often a kit has been used, how can anyone guarantee the kit is ready for the next surgery? With Quest we know everything about the kit, where it is, where it was at a specific date, how often it has been in surgery. It keeps track of the number of surgeries, has the ability to monitor instruments’ lifecycles, and can issue an alert when the kit needs to be returned for inspection and calibration. We also offer a kit reservation system that allows employees to schedule kits in advance.
Imagine being able to collect key data for your field inventory, have the ability to redeploy inventory across territories, make better decisions on what should be consigned or what should be in a loaner pool, make more accurate decisions on how much new inventory needs to be built, and, perhaps most important, bring accountability and management to very expensive assets.
Fenske: You mentioned no interaction is required, whether human or machine input. Does that include the medical professional using the kits within their facility?
Nunes: That’s the beauty of this system. None whatsoever. Once Quest is installed on the kit, it’s live, capturing data, and reporting. The only thing we need to do is conduct a short training on the software. There’s no additional infrastructure requirements, special receivers, or receptors needed. Quest has a proprietary logarithm that not only tells a user where it is located, but it also captures the hospital or customer warehouse location by name.
Fenske: What is the real takeaway for the orthopedic device manufacturer from a business perspective?
Nunes: We often talk about what Quest offers as a new technology. It’s like the difference between being totally blind and having 20/20 vision. I know how much this industry is spending annually on new kits, which, by the way, is the same as the industry’s revenue growth. Inventory turns are deplorable and the industry is behind the times on technology. This industry can’t afford to manage inventory in this way any longer.
Like any other industry, the moment you have real-time, accurate data on a daily basis, the entire business model can be improved because you’re not looking at a snapshot in time. Quest offers more information and capabilities to allow orthopedic leadership to re-invent the entire supply chain and redesign how they interact with customers.
Fenske: With a kit that’s already developed and being deployed by a company, will design adjustments be required before Quest can be used?
Nunes: As we work with each customer, our primary focus is new product launches or new inventory being deployed to the field. The case and tray will be designed with Quest in mind, but we will also have a bracket to secure Quest to existing inventory if the customer chooses to deploy it that way.
QMed is not in the business of selling Quest; we are in the business of solving customer’s inventory management problems with Quest. Each customer will be different and each customer will have their own specific requirements. We are set up to work with each customer on their specific needs. We do not intend to use Quest and the software as a one-size-fits-all approach.
Fenske: So ideally, Quest would be designed into a new product so as to better facilitate the solution?
Nunes: Absolutely. Quest is only the start; we are well aware there are plenty of opportunities for a complete “smart kit” with specific instrument data, eventually acquiring data that tracks instrument usage for optimum kit design or, more specifically, surgeon kit preference. There are also logistics opportunities to help alert when a kit is out of decontamination at a hospital so it can be picked up. Further, there are opportunities with automating the kit reservation system to the hospital surgery scheduling system. Our mission is to provide supply chain optimization through connected technology.
Fenske: What are the next steps for QMed Innovations and the Quest system?
Nunes: In the very short term, educate the industry on our technology. We have developed a very simple business model that allows complete visibility each step of the way of the sales process. In other words, we will develop a complete ROI with the customer, then develop a customer-specific pilot program at a very affordable cost before any significant investment is made. The ROI is simple, but it will help customers realize the added benefits Quest brings.
We are aware of the skepticism within the industry regarding technology and its abilities to address inventory management. As I mentioned, I come from the industry and I was part of leadership teams frustrated with the lack of complete systems to help us better manage our assets. I get it.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Nunes: The way the orthopedic industry conducts business and its relationship with customers has not changed and everyone’s model is the virtually the same. This includes the management of inventory (i.e., consignment, trunk stock, or loaner); interaction with customers (whether distributor or dedicated sales force); the role of the sales rep in the field; how much time they spend on non-selling activities such as logistics and chasing down kits; and management of the instrument lifecycle.
Quest and the data it provides opens the door to change the paradigm of how each orthopedic company will do business and its relationship with the customer. Data and technology is lacking in this industry; without it, business is stagnant. We’re trying to do our part to introduce this type of technology to the industry to cause a paradigm shift in inventory management.
To learn more about Quest and QMed Innovations, visit the company at www.qmedinnovations.com.