Michael Barbella, Managing Editor11.18.20
It’s been quite a year.
Truthfully, it’s been a defining year, though the delineation is rather subjective. For some folks, 2020 has been soul-crushing; for others, it’s been a non-stop frightfest. The journey has been frustrating, depressing, maddening, and confusing at times. But it’s also been a life-altering one.
Certainly, 2020 has worn many labels (none of them good): the Lost Year, the Year Best Forgotten. The Terrible, Horrible, No Good, Very Bad Year. The Worst Year Ever.
One adjective, however, remains missing from that list—Good. That word has (thus far) never been associated with 2020, and it probably never will, thanks to the deadly coronavirus. But rather than dwell on the pandemic and its pilfering of normalcy from daily life, perhaps society—led by the medtech industry—should learn from this health crisis and create solutions for the new normal ahead of us. Society should look forward, not back, as Medtronic’s new CEO Geoff S. Martha advises.
“A lot of people keep talking about ‘hey, when are we going to be back to normal?’ I don’t want to go back to normal,” Martha told Boston Consulting Group’s Bob Lavoie during The Virtual Medtech Conference in October. “I’m an optimistic guy, [and] as painful as the pandemic and the social unrest has been—I wouldn’t wish this on anybody. But we’ve learned a lot and I’m convinced ultimately that society will be better for it as well as companies and especially medtech, being in the heart of this healthcare crisis. We should be part of the answer. So I don’t want to go back, I want to go forward. I think we should define a new normal in terms of how medtech corporations can contribute to some of society’s other issues. That new normal is exciting to me. As painful as it’s been over the last couple of months, the new normal is very exciting. I would urge everybody to not forget the lessons learned, reflect on the last couple of months, and look forward and push forward.”
No matter what the future holds.
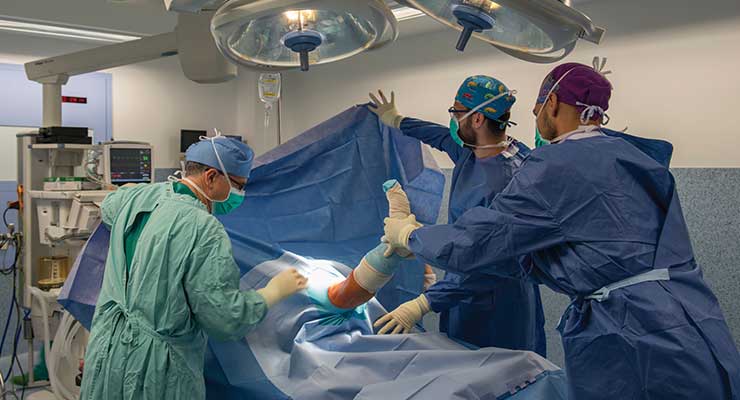
Suspended Operations
His chances were slim, at best, and dwindling by the day.
Yet Roger Best remained hopeful, exuding an optimism beget more by self-preservation instincts than faith.
Best’s sanguinity didn’t last very long, though. As the coronavirus overwhelmed U.S. hospitals in early spring, Best couldn’t help but wonder—and worry—about his upcoming surgery. However, it wasn’t the procedure itself that concerned him, but rather its potential postponement.
The thought of a postponement made Best anxious, for a delay would only prolong his pain. And Best needed the pain to end.
The pain did end, just not as quickly as Best hoped it would.
The tech systems engineer was a week out from his hip replacement surgery in April when NYU Langone Orthopedic Hospital (Manhattan) cancelled it. “A week before the procedure, I was notified I had to reschedule,” the Brooklyn Heights resident recalled to The New York Post. “When they cancelled it, I said to myself, ‘Oh no.’”
Oh, yes. Best was among the millions of patients forced to relinquish their hospital beds to critically ill COVID-19 patients during the pandemic’s initial assault. As the U.S. caseload skyrocketed in late March, states issued executive orders suspending “non-essential” elective surgeries like colonoscopies, endoscopies, non-critical angioplasties/transplants, and hip, knee, and spine procedures.
Orthopedic surgeries were most impacted by the postponements, as such procedures are largely optional. Musculoskeletal repair, in fact, comprised more than one-quarter (26 percent) of the estimated 28.4 million elective procedures sacrificed this year (globally) to SARS-CoV-2. CovidSurge Collaborative data projects an 82 percent cancellation rate for orthopedic surgeries worldwide, with 6.29 million procedures lost to the pandemic.
“The COVID-19 pandemic has had an unprecedented global impact on the way healthcare is delivered,” AAOS Second Vice President Felix H. “Buddy” Savoie III, M.D., FAAOS, told Orthopedic Design & Technology. “The decision to postpone elective surgeries in an effort to limit exposure to the virus, preserve PPE, and reduce the burden on healthcare facility resources and personnel created a radical shift in how orthopedic surgeons provided routine planned care. The halt on elective surgery was far-reaching, affecting physicians, hospitals, and orthopedic implant companies.”
The latter group faced particularly dire consequences, with artificial joint manufacturers reporting massive Q2 earnings losses. Stryker Corporation’s hip and knee sales, for example, were down 37 percent and 45 percent respectively, while its spine revenue sank 39 percent. The damage was equally as staggering at rivals DePuy Synthes Inc., Globus Medical Inc., and Zimmer Biomet Holdings Inc. (De- Puy’s knee proceeds fell by more than half).
The hemorrhaging subsided slightly in Q3 as waning virus caseloads and rescheduled elective procedures boosted demand for orthopedic products and services. Stryker’s knee sales climbed 2 percent, DePuy’s hip revenue rose 2.4 percent, and Globus Medical’s biologics proceeds rose 16 percent; Globus also reported double-digit gains in spine and trauma, and an October sales spike for its ExcelsiusGPS surgical robot.
Globus executives attributed the firm’s stellar financial performance to a “bounce back” in elective procedure volumes as well as continued R&D investment, competitive recruiting/onboarding, and a focused ExcelsiusGPS sales approach. Management is hoping the strategy will return the company to profitability by year’s end.
“I can tell you anecdotally, we’ve certainly got a bounce back,” President and CEO Dave Demski boasted to analysts during a Q3 earnings conference call. “We started seeing a real uptick in procedural volume [in] June, and then that continued through the entire third quarter, which is kind of unusual from a seasonality standpoint. So clearly, a lot of that activity was because of the bounce back from the second quarter.”
That “bounce back” ricocheted through the industry once elective surgeries resumed in May, though the timing and ramp-up speed of those procedures varied by region, institution, and virus caseload. Many hospitals restored volume quickly; others proceeded cautiously, heedful of restart guidelines issued the previous month by numerous medical groups. Some navigated intermittent restarts, their plans sidetracked by COVID-19 resurgences (Alabama, Arizona, Florida, Nevada, South Carolina, and Texas), while a good number juggled elective procedures and spiraling coronavirus cases simultaneously.
Further complicating elective surgery reboots and overall procedure volume was patients’ general trepidation toward entering the O.R. during a pandemic. Case in point: Only 12 percent of Belgians rescheduled their cancelled surgeries in the month after lockdown restrictions ended, an International Orthopaedics study concluded. The data also showed that most patients (90 percent) comprehended the postponement rationale, but 18 percent were nonetheless reluctant to undergo an elective procedure with the virus still a threat.
That reluctance was rather ubiquitous as lockdowns ended in the spring. Less than 20 percent of patients surveyed by Vanderbilt University Medical Center, for example, were willing to undergo elective procedures immediately, with the majority (63-70 percent) planning to wait at least four weeks before scheduling a surgery. Nearly 10 percent of respondents intended to wait longer than six months to resume care for existing non-urgent conditions, the survey data concluded.
“Medical centers have had to prioritize identifying and caring for patients with COVID-19-related illnesses,” an NEJM Catalyst Innovations in Care Delivery report stated. “Around the world, efforts such as social distancing and stay-at-home restrictions have been successful in flattening the COVID-19 curve and decreasing the rate of new cases. The risk of contracting COVID-19 will persist as we divert health care resources back to elective care and will cause fear and anxiety among patients...the survey responses demonstrate hesitancy about routine care and even more so for undergoing elective procedures and surgeries.”
Such hesitancy is bound to exacerbate the backlog of elective procedures, which could last for years depending on capacity levels, care prioritization, coronavirus caseloads, and patient attitudes. Clearing the logjam could take anywhere from 30 weeks to 90 weeks based on British Journal of Surgery estimates of 10-30 percent increases in surgical volume.
Similarly, a May study published in the Journal of Bone and Joint Surgery forecast a U.S. backlog of more than 1 million total joint and spine procedures by mid-2022, with work-through calculations ranging from seven months (50 percent volume growth) and 12 months (30 percent volume increase) to 16 months (20 percent volume hike).
Those kinds of estimations practically guarantee long wait times for future surgeries.
Thankfully, Best won’t be among those lost amid the backup. He had his hip replaced on June 16, barely a week after New York Gov. Andrew Cuomo lifted the state’s elective surgery ban.
“I wanted to get it over as quickly as possible,” Best, 54, explained to the Post. “If a second wave of the coronavirus comes, then what happens? I’m glad I got it done now. You don’t know what the future holds.”
Especially not with the virus permanently part of the future.
Read more: bit.ly/38fBENF
Unleashing the Genie
Yet even as they walk through the valley of the shadow of death, chief executives and corporate strategists are beginning to look to the post-covid world to come. What they think they see, for good or ill, is an acceleration... — The Economist, April 11, 2020
The chatter was disappointing, to say the least.
Earlier this year—ostensibly a lifetime ago now—Eric Jensen journeyed to the West Coast with high hopes for the telehealth industry. A promising but vastly underutilized resource, telehealth solutions have proven effective in combating the shortcomings of cost, quality, and access ingrained in American medicine. The technology’s use has largely been limited though, stifled by patient privacy concerns, strict regulations, financial roadblocks, and scant clinical evidence.
Nevertheless, Jensen was optimistic as he headed West. He knew the sector had experienced exponential growth over the last 15 years, with virtual doctor visits increasing nearly 1,000-fold between 2005 and 2017, and hospital (telehealth) usage more than doubling from 2010 to 2019. He also was aware of the investment surge that funneled roughly $5.5 billion into telehealth services during the 2010s, a decade in which the global market swelled to $45 billion.
Surely such growth should count for something, Jensen reckoned. It should at least help validate telehealth’s role in the total care continuum, and remove the barriers to its widespread adoption. Shouldn’t it?
Perhaps, but telehealth’s skyrocketing growth did not have the impact Jensen was expecting. “...there was still more talk than action,” he wrote in a springtime guest post for healthcare technology news website HIT Consultant. In his post, Jensen described the undertone of uncertainty that shaped digital health discussions at the J.P. Morgan Healthcare Conference in San Francisco. Jensen attended the event in his role as chief product officer for digital health consultancy AVIA.
“...in January 2020, digital [health] still inspired discussion and debate, not investment and action,” he wrote. “Leaders and boards of directors worriedly asked, ‘how might physicians respond to these changes?’ ‘How will digital solutions impact near-term revenue?’ ‘How will we fund these solutions?’ ‘How will these solutions integrate with our EMR?’ And they dithered.”
Not for long, though. The dithering died within weeks of the J.P. Morgan conference as COVID-19 ran amok worldwide, turning telehealth into a societal darling practically overnight.
There was no going back at that point. As Jensen noted in his post, “the genie [was] out of the bottle.”
Released courtesy of Uncle Sam, of course: Federal agencies and public payers scrambled to expand access to telehealth during the pandemic’s initial U.S. assault, removing regulatory and administrative roadblocks, and distributing funding for health IT infrastructure development.
In early March, for example, the Trump administration allowed Medicare to temporarily reimburse telehealth appointments in proportion to office visits, thereby expanding the number of reimbursable virtual care services (144 as of mid-October). Several weeks later, Congress passed a $2.2 billion stimulus bill that benchmarked $200 million for the development of a comprehensive telehealth infrastructure, IT networks, and hardware.
Around the same time, the Centers for Medicare and Medicaid Services (CMS) boosted payments for audio-only telehealth visits and permitted licensed providers to treat patients in different states. In addition, the government relaxed its enforcement of HIPAA privacy and security rules, which had long been hampering telehealth’s widespread use.
CMS also released a new toolkit during the spring to expedite the state adoption of virtual care coverage policies. The toolkit is intended to help states identify potential telehealth services and the regulatory restrictions preventing their quick deployment.
So many changes. So short a time.
Extremely short, actually. In a matter of weeks, the telehealth industry had gone main- stream, bolstered by expedited regulatory shifts and near-instant consumer adoption.
“There are generations where nothing happens. There are decades where nothing happens. And there are weeks when decades happen,” Amwell CEO Roy Schoenberg told Healthcare Dive in July. “And that’s exactly what we’ve gone through.”
In those decades-rich weeks, telehealth services skyrocketed: The proportion of virtual primary care visits among Medicare patients swelled from 0.1 percent in February to 43.5 percent in April, and more than 10.1 million beneficiaries accessed their care remotely between mid-March and early July, government data show.
CMS records paint a similar picture—over 34.5 million Medicaid and CHIP (Children’s Health Insurance Policy) services delivered remotely between March and June, an increase of more than 2,600 percent compared with the same period in 2019. Adults aged 19-64 received the most virtual services, though substantial variations existed across age groups and states, the statistics indicate.
Telehealth providers like Amwell, GlobalMed, Teladoc Health Inc., and Zipnosis nurtured the industry’s meteoric rise with their on-demand platforms and asynchronous offerings. Such innovations helped overwhelmed healthcare systems manage surging demand for virtual services in early spring, when the number of remote care visits grew proportionally to the rising U.S. caseload. MedStar Health’s volume, as an example, spiked from about two daily telehealth visits to 4,150 in two months, and its remote care case total surpassed 100,000 in just 48 days (March 13-May 1).
Similarly, NYU Langone Health conducted 144,940 remote care visits with 115,789 patients in a six-week period (March 2-April 14), according to study data published in the Journal of the American Informatics Association. The numbers show virtual urgent care visits ballooned 683 percent over those six weeks, and non-urgent care appointments swelled an astounding 4,345 percent.
“COVID-19 has fueled a rapid transformation, with telehealth and virtual care driving the new paradigm in care delivery,” American Telemedicine Association (ATA) President Joseph C. Kvedar, M.D., told a U.S. Senate committee on June 17. “This pandemic has forced America’s healthcare system into the 21st century. Telehealth has not been merely a novelty; telehealth has kept the entire healthcare system afloat and has enabled patients to continue to receive care. As technology has advanced, so too has healthcare innovation, creating new and better ways to connect patients and providers, empower individuals to manage their health better, and create more efficient and effective care and improved clinical outcomes. Even just a few short months ago, we could not have anticipated a public health emergency of this magnitude, nor the role telehealth would play in helping to ‘flatten the curve’ while delivering care to millions of Americans.”
In blindsiding the world, COVID-19 exposed some longstanding weaknesses in the U.S. healthcare system. Besides supply chain, testing, data sharing, and communication challenges, SARS-CoV-2 unmasked the financial, cultural, and physical barriers hampering widespread care access.
Telehealth removed at least one major hurdle—namely, helping improve patients’ and doctors’ overall comfort level with virtual visits. A study published in September in Telemedicine and e-Health found that 70.3 percent of orthopedic surgeons were satisified using telemedicine; of the 268 survey respondents, 75 percent reported using the technology for new patients, 86.6 percent use it for routine follow-up patients, and 80.8 percent deploy it for postoperative patients. The data also showed surgeon satisfaction with building rapport and performing meaningful physical exams in established follow-up or postoperative patients.
By mid-June, three-quarters of U.S. hospitals were using digital technology to communicate with patients by video, audio, chat, or email, and patients’ use of telehealth technologies had more than quadrupled, Kvedar’s Congressional testimony noted. Interest in telehealth services also spiked, with 76 percent of consumers expressing a future interest in remote care, the testimony read.
“Patients have grown accustomed to the convenience, safety, and quality of remote visits. The overwhelming acceptance and implementation of telehealth during the pandemic—and the significant levels of patient and provider satisfaction—clearly speak to the value of these technologies,” Kvedar said. “Given the patient and provider satisfaction we have seen, I believe many, if not most, providers and patients will want to continue to use telehealth in some way indefinitely.”
Such a future is untenable, however, without further policy changes to ensure permanent, easy access to virtual care. In his testimony, Kvedar outlined the necessary legislative/regulatory steps for making telehealth services permanent. They include:
“These are not the only policy changes that will be required to ensure telehealth can continue post-pandemic, but they are the most immediate federal policies that must be addressed.
Additionally, technology and telehealth infrastructure remain a critical need,” Kvedar said in his testimony. “Essential telehealth services will abruptly end with the national emergency, and beneficiaries who have come to rely on critical services will be forced back into a world with restricted access to convenient, digitally enabled care. We need [to] ensure patients and providers do not go over the telehealth ‘cliff’ as our nation emerges from the pandemic.”
Indeed, for the fall could be crippling.
Read more: bit.ly/2Hgkyno
Payment Policy Protests
Let the countdown begin.
Like most everyone else on the planet, orthopedic surgeons are eagerly anticipating this year’s demise. And who can blame them? The global pandemic derailed their livelihood as it chased patients from operating rooms and clinics, and stifled the advancements of young arthroplasty residents. Their old world was gone, perhaps for good—the days of handshake greetings, bare-faced meetings, and close patient contact replaced by social distancing, public face coverings, constant hand washing, and virtual office visits.
The amount of tectonic change wrought by the pandemic most certainly has spawned a global hatred for 2020 and a sense of hope for 2021. Yet there is no guarantee the atrocities will ease up next year—anything can occur, really. “Now it’s all about 2021—the year when everything, and maybe nothing, happens,” Associated Press editor Sophia Rosenbaum wrote in mid-July. “No one knows what 2021 will bring. Nobody knows what the ‘new normal’ will look like. There are no clear answers. But there is hope. Every calendar year brings a cycle of hope.”
Embedded in that cycle, however, are a few harsh realities. Orthopedic surgeons anxious to put 2020 behind them must be mindful that the new year will bring further spikes in coronavirus caseloads, continued PPE scarcity, a growing backlog of joint replacement procedures, and Medicare payment policy changes.
Surgeons’ finances might be a bit more strained next year under the changes proffered by the Centers for Medicare & Medicaid Services (CMS). Under the Calendar Year 2021 Hospital Outpatient Prospective Payment System (OPPS) rule, CMS proposes to eliminate the Inpatient Only List, beginning with nearly 300 musculoskeletal-related services. The agency also wants to adjust the criteria for procedures covered in ambulatory settings, and remove certain restrictions on physician-owned hospitals.
Though encouraged by the OPPS rule’s flexibilities, the American Academy of Orthopaedic Surgeons (AAOS) urged CMS to reconsider eliminating the Inpatient Only List, claiming the move would exacerbate unresolved issues and lead to misinterpretation of the policy change.
“This is a complicated clinical and policy decision, and we urge the agency to consider the associated risks to Medicare beneficiaries before finalizing this drastic proposal,” AAOS President Joseph A. Bosco III, M.D., FAAOS, wrote in an Oct. 1 letter to CMS Administrator Seema Verma. “As we have stated in previous comments, AAOS supports the removal of certain procedures from the IPO for which there is evidence that they can safely be performed in the outpatient setting, such as total shoulder arthroplasty and total ankle arthroplasty. We agree with the agency that with developments in the practice of medicine, these procedures can safely be done in the outpatient setting. The AAOS believes that determining the appropriate setting of care should be done through the lens of patient safety and peer-reviewed evidence, and that physicians are best qualified for leading this individualized decision-making process with their patients.”
The AAOS also took issue with CMS’s proposal to cut orthopedic surgical services reimbursement by roughly 5 percent and reduce the work relative value units (RVU) for hip and knee arthroplasty by an additional 5.4 percent beginning Jan. 1.
The physician fee schedule change arose from an American Medical Association committee recommendation to reduce by slightly more than one the RVU used to measure total joint arthroplasty value. AAOS and other industry organizations contend the change devalues orthopedic care and negatively impacts surgeons’ finances.
AAOS petitioned CMS to set specific criteria for determining reimbursable outpatient procedures, and to abandon the two-midnight rule, “which continues to be a source of confusion for hospitals and private payers and an administrative burden for physicians.”
The American Association of Hip and Knee Surgeons (AAHKS) attributed the idea for the physician fee schedule change to a commercial insurance company that reportedly exploited the CMS public nomination process for potentially misvalued codes, possibly to drive down reimbursement to contracted physicians who are paid a percentage of Medicare rates. The evaluation of these codes noted a reduction in physicians’ post-operative time due to emerging efficiencies under value-based care arrangements, but did not recognize corresponding increases in physicians’ pre-operative time which has successfully improved clinical outcomes for hip and knee replacement patients.
The AAHKS said the rule change sends mixed messages to physicians, as the federal government is both attempting to ease regulatory burdens and deliver health provider relief but undermining its efforts by cutting doctors’ payments.
“If these Medicare cuts are finalized, it sends a strong signal: when providers in the vanguard of value-based care begin to achieve some efficiencies in the delivery of care, CMS will use those positive developments as a justification to cut Medicare fee-for-service reimbursement regardless of the extra work that goes into achieving these outcomes,” AAHKS president C. Lowry Barnes, M.D., stated in August.
AAOS fears the 2021 physician fee schedule will affect older Americans’ access to life-improving surgery.
“The AAOS is extremely disappointed in CMS’ decision to disregard our petitioning, many discussions and data presented against these cuts,” an Aug. 5 statement read. “Devaluing the time and effort that orthopaedic surgeons spend prioritizing value-based care communicates a larger plan by the agency to gradually reduce the value of these procedures. Not to mention the fact our surgeons have the highest participation rates across medical subspecialties in alternative payment models, where they work to optimize care and improve patient outcomes all while reducing costs.
“Worsening the financial strain on these practices, at a time when they have been disproportionately affected by COVID-19 federal guidelines to delay care, will have a severe and lasting impact on American seniors’ access to these life-improving surgeries. The AAOS urges CMS to reconsider the significant preoperative work that is required to make value-based care both cost-effective and high-quality, and to refrain from finalizing both of these punitive cuts on the value of orthopaedic care.”
Doing so might give the industry some much-needed hope for 2021.
Read more: bit.ly/354MCU4
Fragmented Finances
All was well at first.
The medtech industry welcomed the new year—and decade—on solid financial footing, with rising total revenue, high investor confidence, and double-digit R&D growth. Strong dealmaking was in the forecast, particularly within digital health. And, the bothersome medical device tax was (finally) no longer a threat, thanks to a rare yuletide miracle from Uncle Sam.
Indeed, 2020 looked promising. But that optimism quickly faded as COVID-19 circled the globe, upending society with government-imposed shutdowns and social distancing measures. Global stock markets crashed (the Dow lost 30 percent of its value in just a few weeks), unemployment skyrocketed, and economic activity slowed to a trickle. The pecuniary carnage that followed ended America’s historic 128-month expansion and forced the world into recession.
The volatility utterly decimated orthopedic finances: Stryker Corp.’s second-quarter sales fell 36 percent, with knees revenue plummeting 45.2 percent and hips sinking 37 percent. Similarly, Smith+Nephew’s Q2 proceeds tumbled 29.3 percent (its Orthopaedics franchise posted a 34 percent loss), NuVasive Inc.’s revenue slid 30.3 percent, and Zimmer Biomet Holdings Inc. posted a 38.3 percent deficit in net sales.
“It’s clear that the challenge of COVID-19 has been significant to Zimmer Biomet—to many, obviously,” President and CEO Bryan Hanson told analysts on an earnings conference call in early August. “...when we think about variables, that idea of the headwinds being patient fear and recurrence of the virus, those are big variables, and they’re going to be unpredictable, unfortunately. There’s no way to predict what they’re going to do in the future.”
Such volatility kept profits mostly at bay this year. Despite solid third-quarter gains, roughly two-thirds of pure-play medtech companies and conglomerates lost 5 percent in total revenue in H1 2020, according to EY report data. IPO valuations were up, but the number of offerings fell to their lowest level in a decade (14), and venture capital investments contracted 22 percent (the second quarter was particularly disastrous).
Much of the surviving venture activity involved non-imaging diagnostics innovation. M&A was fairly muted this year too, as total deal value and transactions declined from 2019 levels. Expenditures between July 2019 and June 2020 plummeted 60 percent to $27.1 billion compared to the previous 12-month period, EY statistics indicate. Similarly, the total number of deals in the first half of 2020 sank 83 percent to 19, Capstone Headwaters analysts claim.
The year’s largest acquisition by far was Siemens Healthineers’ $16.4 billion takeover of Palo Alto, Calif.-based Varian Medical Systems. Announced in August, the all-cash deal gives Siemens a suite of cancer treatment technologies, including a growing proton therapy business.
The Siemens-Varian union was medtech’s only big-name pairing in 2020, as Stryker’s $4 billion bid for Wright Medical Group stalled amid an entanglement of red tape. First announced in November 2019, the acquisition has drawn scrutiny this year from Wright Medical shareholders, the U.S. Federal Trade Commission (FTC), and the U.K.’s Competition and Markets Authority (CMA).
Shareholders voiced their objection to the deal in mid-January via a class-action lawsuit that accuses Stryker of failing to disclose deal-related financial projections. The FTC and CMA both took issue with the acquisition’s potential market impacts; consequently, the CMA suggested in July that Stryker divest its Scandinavian Total Ankle Replacement (STAR) product line, and the FTC followed suit in early November by requiring the company sell all its total ankle replacement assets and finger joint implant products.
Compounding the dearth of blockbuster acquisitions this year was a decline in both non-megadeal expenditures and deal value. Total non-megadeal expenditures fell 41 percent (July 2019-June 2020) and average deal value declined to $167 million (from $463 million in the previous 12-month period), EY statistics show. Certainly, many smaller transactions occurred in the year’s first 10 months, including Anika Therapeutics Inc.’s $100 million buyout of Arthrosurface, Conventus Orthopaedics’ acquisition of sterile packaged implant/instrument maker Flower Orthopedics, Spineart’s merger with Meditech Spine, and OrthoPediatrics Corp.’s purchase of ApiFix Ltd.
“ApiFix fills a major treatment gap that could potentially allow patients to avoid fusion surgery. We estimate that non-fusion procedures will grow significantly as patients, their families, and surgeons recognize non-fusion’s benefits,” OrthoPediatrics President and CEO Mark Throdahl noted in announcing the April deal. “The ApiFix system has a number of advantages over vertebral body tethering—it is significantly less complex and risky, does not imply the need for a thoracic or general surgeon, and has fewer complications. The acquisition of this technology keeps OrthoPediatrics at the forefront of pediatric orthopedic care with a viable alternative to failed bracing and spinal fusion for the treatment of progressive scoliosis.”
To remain at the forefront of pediatric care, OrthoPediatrics in January divested its Vilex adult product offering, which consisted of staples, screws, plates, joint implants, and fusion devices. That same month, RTI Surgical Holdings shed its OEM business to become a pure-play global spine company.
Many orthopedic companies turned to M&A this year to expand their market reach or product portfolios. Anika Therapeutics, for example, gained access to ambulatory surgery centers by purchasing Parcus Medical, and WishBone Medical Inc. infiltrated the pediatric spine market with the buyout of Back 2 Basics Direct LLC and Orbbö Surgical LLC.
Medtronic, meanwhile, bolstered its spinal surgery offerings through its Medicrea purchase; Vilex LLC augmented its extremity trauma device capabilities via its DT Medtech LLC buyout; Wenzel Spine inherited diagnostic and decision-making tools from Statera Spine; and Smith+Nephew enhanced its extremities lineup courtesy of Integra Lifesciences’ discarded extremities business.
M&A accelerated somewhat in the second half of 2020, due largely to the pandemic (ironically), which analysts say is creating a buyer’s market among medtech firms that doubt they can survive the virus-induced economic uncertainty. Larger companies, in the meantime, have recapitalized through debt and follow-on offerings, and now have substantial MA& firepower, according to EY.
“The industry anticipates an accelerated growth cycle, with companies valued at $30 million to $40 million becoming targets,” John Babitt, EY Americas Strategy and Transactions Medtech leader, suggests in the pulse of the industry report. “...if we as an industry get some sense of normalcy into the fall, the high level of available capital could trigger an M&A acceleration.”
But that’s a big if, particularly in a highly abnormal year.
Read more: bit.ly/38m9ost
Truthfully, it’s been a defining year, though the delineation is rather subjective. For some folks, 2020 has been soul-crushing; for others, it’s been a non-stop frightfest. The journey has been frustrating, depressing, maddening, and confusing at times. But it’s also been a life-altering one.
Certainly, 2020 has worn many labels (none of them good): the Lost Year, the Year Best Forgotten. The Terrible, Horrible, No Good, Very Bad Year. The Worst Year Ever.
One adjective, however, remains missing from that list—Good. That word has (thus far) never been associated with 2020, and it probably never will, thanks to the deadly coronavirus. But rather than dwell on the pandemic and its pilfering of normalcy from daily life, perhaps society—led by the medtech industry—should learn from this health crisis and create solutions for the new normal ahead of us. Society should look forward, not back, as Medtronic’s new CEO Geoff S. Martha advises.
“A lot of people keep talking about ‘hey, when are we going to be back to normal?’ I don’t want to go back to normal,” Martha told Boston Consulting Group’s Bob Lavoie during The Virtual Medtech Conference in October. “I’m an optimistic guy, [and] as painful as the pandemic and the social unrest has been—I wouldn’t wish this on anybody. But we’ve learned a lot and I’m convinced ultimately that society will be better for it as well as companies and especially medtech, being in the heart of this healthcare crisis. We should be part of the answer. So I don’t want to go back, I want to go forward. I think we should define a new normal in terms of how medtech corporations can contribute to some of society’s other issues. That new normal is exciting to me. As painful as it’s been over the last couple of months, the new normal is very exciting. I would urge everybody to not forget the lessons learned, reflect on the last couple of months, and look forward and push forward.”
No matter what the future holds.
Suspended Operations
His chances were slim, at best, and dwindling by the day.
Yet Roger Best remained hopeful, exuding an optimism beget more by self-preservation instincts than faith.
Best’s sanguinity didn’t last very long, though. As the coronavirus overwhelmed U.S. hospitals in early spring, Best couldn’t help but wonder—and worry—about his upcoming surgery. However, it wasn’t the procedure itself that concerned him, but rather its potential postponement.
The thought of a postponement made Best anxious, for a delay would only prolong his pain. And Best needed the pain to end.
The pain did end, just not as quickly as Best hoped it would.
The tech systems engineer was a week out from his hip replacement surgery in April when NYU Langone Orthopedic Hospital (Manhattan) cancelled it. “A week before the procedure, I was notified I had to reschedule,” the Brooklyn Heights resident recalled to The New York Post. “When they cancelled it, I said to myself, ‘Oh no.’”
Oh, yes. Best was among the millions of patients forced to relinquish their hospital beds to critically ill COVID-19 patients during the pandemic’s initial assault. As the U.S. caseload skyrocketed in late March, states issued executive orders suspending “non-essential” elective surgeries like colonoscopies, endoscopies, non-critical angioplasties/transplants, and hip, knee, and spine procedures.
Orthopedic surgeries were most impacted by the postponements, as such procedures are largely optional. Musculoskeletal repair, in fact, comprised more than one-quarter (26 percent) of the estimated 28.4 million elective procedures sacrificed this year (globally) to SARS-CoV-2. CovidSurge Collaborative data projects an 82 percent cancellation rate for orthopedic surgeries worldwide, with 6.29 million procedures lost to the pandemic.
“The COVID-19 pandemic has had an unprecedented global impact on the way healthcare is delivered,” AAOS Second Vice President Felix H. “Buddy” Savoie III, M.D., FAAOS, told Orthopedic Design & Technology. “The decision to postpone elective surgeries in an effort to limit exposure to the virus, preserve PPE, and reduce the burden on healthcare facility resources and personnel created a radical shift in how orthopedic surgeons provided routine planned care. The halt on elective surgery was far-reaching, affecting physicians, hospitals, and orthopedic implant companies.”
The latter group faced particularly dire consequences, with artificial joint manufacturers reporting massive Q2 earnings losses. Stryker Corporation’s hip and knee sales, for example, were down 37 percent and 45 percent respectively, while its spine revenue sank 39 percent. The damage was equally as staggering at rivals DePuy Synthes Inc., Globus Medical Inc., and Zimmer Biomet Holdings Inc. (De- Puy’s knee proceeds fell by more than half).
The hemorrhaging subsided slightly in Q3 as waning virus caseloads and rescheduled elective procedures boosted demand for orthopedic products and services. Stryker’s knee sales climbed 2 percent, DePuy’s hip revenue rose 2.4 percent, and Globus Medical’s biologics proceeds rose 16 percent; Globus also reported double-digit gains in spine and trauma, and an October sales spike for its ExcelsiusGPS surgical robot.
Globus executives attributed the firm’s stellar financial performance to a “bounce back” in elective procedure volumes as well as continued R&D investment, competitive recruiting/onboarding, and a focused ExcelsiusGPS sales approach. Management is hoping the strategy will return the company to profitability by year’s end.
“I can tell you anecdotally, we’ve certainly got a bounce back,” President and CEO Dave Demski boasted to analysts during a Q3 earnings conference call. “We started seeing a real uptick in procedural volume [in] June, and then that continued through the entire third quarter, which is kind of unusual from a seasonality standpoint. So clearly, a lot of that activity was because of the bounce back from the second quarter.”
That “bounce back” ricocheted through the industry once elective surgeries resumed in May, though the timing and ramp-up speed of those procedures varied by region, institution, and virus caseload. Many hospitals restored volume quickly; others proceeded cautiously, heedful of restart guidelines issued the previous month by numerous medical groups. Some navigated intermittent restarts, their plans sidetracked by COVID-19 resurgences (Alabama, Arizona, Florida, Nevada, South Carolina, and Texas), while a good number juggled elective procedures and spiraling coronavirus cases simultaneously.
Further complicating elective surgery reboots and overall procedure volume was patients’ general trepidation toward entering the O.R. during a pandemic. Case in point: Only 12 percent of Belgians rescheduled their cancelled surgeries in the month after lockdown restrictions ended, an International Orthopaedics study concluded. The data also showed that most patients (90 percent) comprehended the postponement rationale, but 18 percent were nonetheless reluctant to undergo an elective procedure with the virus still a threat.
That reluctance was rather ubiquitous as lockdowns ended in the spring. Less than 20 percent of patients surveyed by Vanderbilt University Medical Center, for example, were willing to undergo elective procedures immediately, with the majority (63-70 percent) planning to wait at least four weeks before scheduling a surgery. Nearly 10 percent of respondents intended to wait longer than six months to resume care for existing non-urgent conditions, the survey data concluded.
“Medical centers have had to prioritize identifying and caring for patients with COVID-19-related illnesses,” an NEJM Catalyst Innovations in Care Delivery report stated. “Around the world, efforts such as social distancing and stay-at-home restrictions have been successful in flattening the COVID-19 curve and decreasing the rate of new cases. The risk of contracting COVID-19 will persist as we divert health care resources back to elective care and will cause fear and anxiety among patients...the survey responses demonstrate hesitancy about routine care and even more so for undergoing elective procedures and surgeries.”
Such hesitancy is bound to exacerbate the backlog of elective procedures, which could last for years depending on capacity levels, care prioritization, coronavirus caseloads, and patient attitudes. Clearing the logjam could take anywhere from 30 weeks to 90 weeks based on British Journal of Surgery estimates of 10-30 percent increases in surgical volume.
Similarly, a May study published in the Journal of Bone and Joint Surgery forecast a U.S. backlog of more than 1 million total joint and spine procedures by mid-2022, with work-through calculations ranging from seven months (50 percent volume growth) and 12 months (30 percent volume increase) to 16 months (20 percent volume hike).
Those kinds of estimations practically guarantee long wait times for future surgeries.
Thankfully, Best won’t be among those lost amid the backup. He had his hip replaced on June 16, barely a week after New York Gov. Andrew Cuomo lifted the state’s elective surgery ban.
“I wanted to get it over as quickly as possible,” Best, 54, explained to the Post. “If a second wave of the coronavirus comes, then what happens? I’m glad I got it done now. You don’t know what the future holds.”
Especially not with the virus permanently part of the future.
Read more: bit.ly/38fBENF
Unleashing the Genie
Yet even as they walk through the valley of the shadow of death, chief executives and corporate strategists are beginning to look to the post-covid world to come. What they think they see, for good or ill, is an acceleration... — The Economist, April 11, 2020
The chatter was disappointing, to say the least.
Earlier this year—ostensibly a lifetime ago now—Eric Jensen journeyed to the West Coast with high hopes for the telehealth industry. A promising but vastly underutilized resource, telehealth solutions have proven effective in combating the shortcomings of cost, quality, and access ingrained in American medicine. The technology’s use has largely been limited though, stifled by patient privacy concerns, strict regulations, financial roadblocks, and scant clinical evidence.
Nevertheless, Jensen was optimistic as he headed West. He knew the sector had experienced exponential growth over the last 15 years, with virtual doctor visits increasing nearly 1,000-fold between 2005 and 2017, and hospital (telehealth) usage more than doubling from 2010 to 2019. He also was aware of the investment surge that funneled roughly $5.5 billion into telehealth services during the 2010s, a decade in which the global market swelled to $45 billion.
Surely such growth should count for something, Jensen reckoned. It should at least help validate telehealth’s role in the total care continuum, and remove the barriers to its widespread adoption. Shouldn’t it?
Perhaps, but telehealth’s skyrocketing growth did not have the impact Jensen was expecting. “...there was still more talk than action,” he wrote in a springtime guest post for healthcare technology news website HIT Consultant. In his post, Jensen described the undertone of uncertainty that shaped digital health discussions at the J.P. Morgan Healthcare Conference in San Francisco. Jensen attended the event in his role as chief product officer for digital health consultancy AVIA.
“...in January 2020, digital [health] still inspired discussion and debate, not investment and action,” he wrote. “Leaders and boards of directors worriedly asked, ‘how might physicians respond to these changes?’ ‘How will digital solutions impact near-term revenue?’ ‘How will we fund these solutions?’ ‘How will these solutions integrate with our EMR?’ And they dithered.”
Not for long, though. The dithering died within weeks of the J.P. Morgan conference as COVID-19 ran amok worldwide, turning telehealth into a societal darling practically overnight.
There was no going back at that point. As Jensen noted in his post, “the genie [was] out of the bottle.”
Released courtesy of Uncle Sam, of course: Federal agencies and public payers scrambled to expand access to telehealth during the pandemic’s initial U.S. assault, removing regulatory and administrative roadblocks, and distributing funding for health IT infrastructure development.
In early March, for example, the Trump administration allowed Medicare to temporarily reimburse telehealth appointments in proportion to office visits, thereby expanding the number of reimbursable virtual care services (144 as of mid-October). Several weeks later, Congress passed a $2.2 billion stimulus bill that benchmarked $200 million for the development of a comprehensive telehealth infrastructure, IT networks, and hardware.
Around the same time, the Centers for Medicare and Medicaid Services (CMS) boosted payments for audio-only telehealth visits and permitted licensed providers to treat patients in different states. In addition, the government relaxed its enforcement of HIPAA privacy and security rules, which had long been hampering telehealth’s widespread use.
CMS also released a new toolkit during the spring to expedite the state adoption of virtual care coverage policies. The toolkit is intended to help states identify potential telehealth services and the regulatory restrictions preventing their quick deployment.
So many changes. So short a time.
Extremely short, actually. In a matter of weeks, the telehealth industry had gone main- stream, bolstered by expedited regulatory shifts and near-instant consumer adoption.
“There are generations where nothing happens. There are decades where nothing happens. And there are weeks when decades happen,” Amwell CEO Roy Schoenberg told Healthcare Dive in July. “And that’s exactly what we’ve gone through.”
In those decades-rich weeks, telehealth services skyrocketed: The proportion of virtual primary care visits among Medicare patients swelled from 0.1 percent in February to 43.5 percent in April, and more than 10.1 million beneficiaries accessed their care remotely between mid-March and early July, government data show.
CMS records paint a similar picture—over 34.5 million Medicaid and CHIP (Children’s Health Insurance Policy) services delivered remotely between March and June, an increase of more than 2,600 percent compared with the same period in 2019. Adults aged 19-64 received the most virtual services, though substantial variations existed across age groups and states, the statistics indicate.
Telehealth providers like Amwell, GlobalMed, Teladoc Health Inc., and Zipnosis nurtured the industry’s meteoric rise with their on-demand platforms and asynchronous offerings. Such innovations helped overwhelmed healthcare systems manage surging demand for virtual services in early spring, when the number of remote care visits grew proportionally to the rising U.S. caseload. MedStar Health’s volume, as an example, spiked from about two daily telehealth visits to 4,150 in two months, and its remote care case total surpassed 100,000 in just 48 days (March 13-May 1).
Similarly, NYU Langone Health conducted 144,940 remote care visits with 115,789 patients in a six-week period (March 2-April 14), according to study data published in the Journal of the American Informatics Association. The numbers show virtual urgent care visits ballooned 683 percent over those six weeks, and non-urgent care appointments swelled an astounding 4,345 percent.
“COVID-19 has fueled a rapid transformation, with telehealth and virtual care driving the new paradigm in care delivery,” American Telemedicine Association (ATA) President Joseph C. Kvedar, M.D., told a U.S. Senate committee on June 17. “This pandemic has forced America’s healthcare system into the 21st century. Telehealth has not been merely a novelty; telehealth has kept the entire healthcare system afloat and has enabled patients to continue to receive care. As technology has advanced, so too has healthcare innovation, creating new and better ways to connect patients and providers, empower individuals to manage their health better, and create more efficient and effective care and improved clinical outcomes. Even just a few short months ago, we could not have anticipated a public health emergency of this magnitude, nor the role telehealth would play in helping to ‘flatten the curve’ while delivering care to millions of Americans.”
In blindsiding the world, COVID-19 exposed some longstanding weaknesses in the U.S. healthcare system. Besides supply chain, testing, data sharing, and communication challenges, SARS-CoV-2 unmasked the financial, cultural, and physical barriers hampering widespread care access.
Telehealth removed at least one major hurdle—namely, helping improve patients’ and doctors’ overall comfort level with virtual visits. A study published in September in Telemedicine and e-Health found that 70.3 percent of orthopedic surgeons were satisified using telemedicine; of the 268 survey respondents, 75 percent reported using the technology for new patients, 86.6 percent use it for routine follow-up patients, and 80.8 percent deploy it for postoperative patients. The data also showed surgeon satisfaction with building rapport and performing meaningful physical exams in established follow-up or postoperative patients.
By mid-June, three-quarters of U.S. hospitals were using digital technology to communicate with patients by video, audio, chat, or email, and patients’ use of telehealth technologies had more than quadrupled, Kvedar’s Congressional testimony noted. Interest in telehealth services also spiked, with 76 percent of consumers expressing a future interest in remote care, the testimony read.
“Patients have grown accustomed to the convenience, safety, and quality of remote visits. The overwhelming acceptance and implementation of telehealth during the pandemic—and the significant levels of patient and provider satisfaction—clearly speak to the value of these technologies,” Kvedar said. “Given the patient and provider satisfaction we have seen, I believe many, if not most, providers and patients will want to continue to use telehealth in some way indefinitely.”
Such a future is untenable, however, without further policy changes to ensure permanent, easy access to virtual care. In his testimony, Kvedar outlined the necessary legislative/regulatory steps for making telehealth services permanent. They include:
- Addressing currently outdated statutory restrictions on patient geography and originating site limitations;
- Expanding the list of eligible practitioners and therapy services, and maintaining the authority to add or remove specific telehealth services;
- Allowing CMS to determine and manage the range and scope of telehealth services;
- Considering maintaining HIPAA rule flexibility; and
- Encouraging states to continue working together to offer more streamlined licensing across state lines.
“These are not the only policy changes that will be required to ensure telehealth can continue post-pandemic, but they are the most immediate federal policies that must be addressed.
Additionally, technology and telehealth infrastructure remain a critical need,” Kvedar said in his testimony. “Essential telehealth services will abruptly end with the national emergency, and beneficiaries who have come to rely on critical services will be forced back into a world with restricted access to convenient, digitally enabled care. We need [to] ensure patients and providers do not go over the telehealth ‘cliff’ as our nation emerges from the pandemic.”
Indeed, for the fall could be crippling.
Read more: bit.ly/2Hgkyno
Payment Policy Protests
Let the countdown begin.
Like most everyone else on the planet, orthopedic surgeons are eagerly anticipating this year’s demise. And who can blame them? The global pandemic derailed their livelihood as it chased patients from operating rooms and clinics, and stifled the advancements of young arthroplasty residents. Their old world was gone, perhaps for good—the days of handshake greetings, bare-faced meetings, and close patient contact replaced by social distancing, public face coverings, constant hand washing, and virtual office visits.
The amount of tectonic change wrought by the pandemic most certainly has spawned a global hatred for 2020 and a sense of hope for 2021. Yet there is no guarantee the atrocities will ease up next year—anything can occur, really. “Now it’s all about 2021—the year when everything, and maybe nothing, happens,” Associated Press editor Sophia Rosenbaum wrote in mid-July. “No one knows what 2021 will bring. Nobody knows what the ‘new normal’ will look like. There are no clear answers. But there is hope. Every calendar year brings a cycle of hope.”
Embedded in that cycle, however, are a few harsh realities. Orthopedic surgeons anxious to put 2020 behind them must be mindful that the new year will bring further spikes in coronavirus caseloads, continued PPE scarcity, a growing backlog of joint replacement procedures, and Medicare payment policy changes.
Surgeons’ finances might be a bit more strained next year under the changes proffered by the Centers for Medicare & Medicaid Services (CMS). Under the Calendar Year 2021 Hospital Outpatient Prospective Payment System (OPPS) rule, CMS proposes to eliminate the Inpatient Only List, beginning with nearly 300 musculoskeletal-related services. The agency also wants to adjust the criteria for procedures covered in ambulatory settings, and remove certain restrictions on physician-owned hospitals.
Though encouraged by the OPPS rule’s flexibilities, the American Academy of Orthopaedic Surgeons (AAOS) urged CMS to reconsider eliminating the Inpatient Only List, claiming the move would exacerbate unresolved issues and lead to misinterpretation of the policy change.
“This is a complicated clinical and policy decision, and we urge the agency to consider the associated risks to Medicare beneficiaries before finalizing this drastic proposal,” AAOS President Joseph A. Bosco III, M.D., FAAOS, wrote in an Oct. 1 letter to CMS Administrator Seema Verma. “As we have stated in previous comments, AAOS supports the removal of certain procedures from the IPO for which there is evidence that they can safely be performed in the outpatient setting, such as total shoulder arthroplasty and total ankle arthroplasty. We agree with the agency that with developments in the practice of medicine, these procedures can safely be done in the outpatient setting. The AAOS believes that determining the appropriate setting of care should be done through the lens of patient safety and peer-reviewed evidence, and that physicians are best qualified for leading this individualized decision-making process with their patients.”
The AAOS also took issue with CMS’s proposal to cut orthopedic surgical services reimbursement by roughly 5 percent and reduce the work relative value units (RVU) for hip and knee arthroplasty by an additional 5.4 percent beginning Jan. 1.
The physician fee schedule change arose from an American Medical Association committee recommendation to reduce by slightly more than one the RVU used to measure total joint arthroplasty value. AAOS and other industry organizations contend the change devalues orthopedic care and negatively impacts surgeons’ finances.
AAOS petitioned CMS to set specific criteria for determining reimbursable outpatient procedures, and to abandon the two-midnight rule, “which continues to be a source of confusion for hospitals and private payers and an administrative burden for physicians.”
The American Association of Hip and Knee Surgeons (AAHKS) attributed the idea for the physician fee schedule change to a commercial insurance company that reportedly exploited the CMS public nomination process for potentially misvalued codes, possibly to drive down reimbursement to contracted physicians who are paid a percentage of Medicare rates. The evaluation of these codes noted a reduction in physicians’ post-operative time due to emerging efficiencies under value-based care arrangements, but did not recognize corresponding increases in physicians’ pre-operative time which has successfully improved clinical outcomes for hip and knee replacement patients.
The AAHKS said the rule change sends mixed messages to physicians, as the federal government is both attempting to ease regulatory burdens and deliver health provider relief but undermining its efforts by cutting doctors’ payments.
“If these Medicare cuts are finalized, it sends a strong signal: when providers in the vanguard of value-based care begin to achieve some efficiencies in the delivery of care, CMS will use those positive developments as a justification to cut Medicare fee-for-service reimbursement regardless of the extra work that goes into achieving these outcomes,” AAHKS president C. Lowry Barnes, M.D., stated in August.
AAOS fears the 2021 physician fee schedule will affect older Americans’ access to life-improving surgery.
“The AAOS is extremely disappointed in CMS’ decision to disregard our petitioning, many discussions and data presented against these cuts,” an Aug. 5 statement read. “Devaluing the time and effort that orthopaedic surgeons spend prioritizing value-based care communicates a larger plan by the agency to gradually reduce the value of these procedures. Not to mention the fact our surgeons have the highest participation rates across medical subspecialties in alternative payment models, where they work to optimize care and improve patient outcomes all while reducing costs.
“Worsening the financial strain on these practices, at a time when they have been disproportionately affected by COVID-19 federal guidelines to delay care, will have a severe and lasting impact on American seniors’ access to these life-improving surgeries. The AAOS urges CMS to reconsider the significant preoperative work that is required to make value-based care both cost-effective and high-quality, and to refrain from finalizing both of these punitive cuts on the value of orthopaedic care.”
Doing so might give the industry some much-needed hope for 2021.
Read more: bit.ly/354MCU4
Fragmented Finances
All was well at first.
The medtech industry welcomed the new year—and decade—on solid financial footing, with rising total revenue, high investor confidence, and double-digit R&D growth. Strong dealmaking was in the forecast, particularly within digital health. And, the bothersome medical device tax was (finally) no longer a threat, thanks to a rare yuletide miracle from Uncle Sam.
Indeed, 2020 looked promising. But that optimism quickly faded as COVID-19 circled the globe, upending society with government-imposed shutdowns and social distancing measures. Global stock markets crashed (the Dow lost 30 percent of its value in just a few weeks), unemployment skyrocketed, and economic activity slowed to a trickle. The pecuniary carnage that followed ended America’s historic 128-month expansion and forced the world into recession.
The volatility utterly decimated orthopedic finances: Stryker Corp.’s second-quarter sales fell 36 percent, with knees revenue plummeting 45.2 percent and hips sinking 37 percent. Similarly, Smith+Nephew’s Q2 proceeds tumbled 29.3 percent (its Orthopaedics franchise posted a 34 percent loss), NuVasive Inc.’s revenue slid 30.3 percent, and Zimmer Biomet Holdings Inc. posted a 38.3 percent deficit in net sales.
“It’s clear that the challenge of COVID-19 has been significant to Zimmer Biomet—to many, obviously,” President and CEO Bryan Hanson told analysts on an earnings conference call in early August. “...when we think about variables, that idea of the headwinds being patient fear and recurrence of the virus, those are big variables, and they’re going to be unpredictable, unfortunately. There’s no way to predict what they’re going to do in the future.”
Such volatility kept profits mostly at bay this year. Despite solid third-quarter gains, roughly two-thirds of pure-play medtech companies and conglomerates lost 5 percent in total revenue in H1 2020, according to EY report data. IPO valuations were up, but the number of offerings fell to their lowest level in a decade (14), and venture capital investments contracted 22 percent (the second quarter was particularly disastrous).
Much of the surviving venture activity involved non-imaging diagnostics innovation. M&A was fairly muted this year too, as total deal value and transactions declined from 2019 levels. Expenditures between July 2019 and June 2020 plummeted 60 percent to $27.1 billion compared to the previous 12-month period, EY statistics indicate. Similarly, the total number of deals in the first half of 2020 sank 83 percent to 19, Capstone Headwaters analysts claim.
The year’s largest acquisition by far was Siemens Healthineers’ $16.4 billion takeover of Palo Alto, Calif.-based Varian Medical Systems. Announced in August, the all-cash deal gives Siemens a suite of cancer treatment technologies, including a growing proton therapy business.
The Siemens-Varian union was medtech’s only big-name pairing in 2020, as Stryker’s $4 billion bid for Wright Medical Group stalled amid an entanglement of red tape. First announced in November 2019, the acquisition has drawn scrutiny this year from Wright Medical shareholders, the U.S. Federal Trade Commission (FTC), and the U.K.’s Competition and Markets Authority (CMA).
Shareholders voiced their objection to the deal in mid-January via a class-action lawsuit that accuses Stryker of failing to disclose deal-related financial projections. The FTC and CMA both took issue with the acquisition’s potential market impacts; consequently, the CMA suggested in July that Stryker divest its Scandinavian Total Ankle Replacement (STAR) product line, and the FTC followed suit in early November by requiring the company sell all its total ankle replacement assets and finger joint implant products.
Compounding the dearth of blockbuster acquisitions this year was a decline in both non-megadeal expenditures and deal value. Total non-megadeal expenditures fell 41 percent (July 2019-June 2020) and average deal value declined to $167 million (from $463 million in the previous 12-month period), EY statistics show. Certainly, many smaller transactions occurred in the year’s first 10 months, including Anika Therapeutics Inc.’s $100 million buyout of Arthrosurface, Conventus Orthopaedics’ acquisition of sterile packaged implant/instrument maker Flower Orthopedics, Spineart’s merger with Meditech Spine, and OrthoPediatrics Corp.’s purchase of ApiFix Ltd.
“ApiFix fills a major treatment gap that could potentially allow patients to avoid fusion surgery. We estimate that non-fusion procedures will grow significantly as patients, their families, and surgeons recognize non-fusion’s benefits,” OrthoPediatrics President and CEO Mark Throdahl noted in announcing the April deal. “The ApiFix system has a number of advantages over vertebral body tethering—it is significantly less complex and risky, does not imply the need for a thoracic or general surgeon, and has fewer complications. The acquisition of this technology keeps OrthoPediatrics at the forefront of pediatric orthopedic care with a viable alternative to failed bracing and spinal fusion for the treatment of progressive scoliosis.”
To remain at the forefront of pediatric care, OrthoPediatrics in January divested its Vilex adult product offering, which consisted of staples, screws, plates, joint implants, and fusion devices. That same month, RTI Surgical Holdings shed its OEM business to become a pure-play global spine company.
Many orthopedic companies turned to M&A this year to expand their market reach or product portfolios. Anika Therapeutics, for example, gained access to ambulatory surgery centers by purchasing Parcus Medical, and WishBone Medical Inc. infiltrated the pediatric spine market with the buyout of Back 2 Basics Direct LLC and Orbbö Surgical LLC.
Medtronic, meanwhile, bolstered its spinal surgery offerings through its Medicrea purchase; Vilex LLC augmented its extremity trauma device capabilities via its DT Medtech LLC buyout; Wenzel Spine inherited diagnostic and decision-making tools from Statera Spine; and Smith+Nephew enhanced its extremities lineup courtesy of Integra Lifesciences’ discarded extremities business.
M&A accelerated somewhat in the second half of 2020, due largely to the pandemic (ironically), which analysts say is creating a buyer’s market among medtech firms that doubt they can survive the virus-induced economic uncertainty. Larger companies, in the meantime, have recapitalized through debt and follow-on offerings, and now have substantial MA& firepower, according to EY.
“The industry anticipates an accelerated growth cycle, with companies valued at $30 million to $40 million becoming targets,” John Babitt, EY Americas Strategy and Transactions Medtech leader, suggests in the pulse of the industry report. “...if we as an industry get some sense of normalcy into the fall, the high level of available capital could trigger an M&A acceleration.”
But that’s a big if, particularly in a highly abnormal year.
Read more: bit.ly/38m9ost