Kleiner Device Labs09.22.21
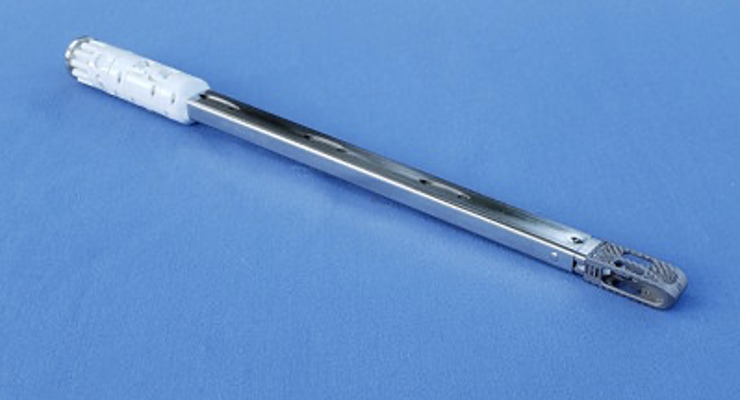
Kleiner Device Labs has received U.S. Food and Drug Administration (FDA) 510(k) market clearance for its KG2 Surge flow-thru interbody system. The system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and grafting process for TLIF and PLIF spinal fusion procedures.
"Getting adequate graft fill volume and distribution of graft within the intervertebral disc space is one of the most vexing challenges in spinal fusion procedures, which led us to develop the unique flow-thru technology in our KG series products," noted Jeff Kleiner, MD, founder and CEO of Kleiner Device Labs. "The new KG2 Surge system integrates graft delivery with the interbody device delivery. It allows the surgeon to first distract the disc space by inserting the implant, and then packing the entire disc space bilaterally through the KG2 Surge implant, using the attached cannulated inserter. Fully packing the graft with the disc space in the distracted and final position ensures graft/endplate contact. The system's large rectangular cannula allows simplified delivery of a wide range of allograft and autograft products, including viscous and fibrous materials."
The implant's unique I-beam architecture and ramp design allow unrestricted flow to bilaterally fill and integrate the implant into the prepared disc space. The KG2 Surge interbody device is 3-D printed titanium and features a diamond lattice porous structure to facilitate bone ingrowth.
The KG2 Surge flow-thru interbody system comes with the interbody device pre-assembled with the cannula in a single-use sterile tray.
Dr. Kleiner added, "The surgeon opens our tray with a ready-to-use system in their hands. The learning curve is low for operating room staff, and the KG2 Surge system works in both MIS and open approaches. The associated reusable instrument package allows for immediate revision at every step, should the surgeon desire implant repositioning or removal."
KG2 Surge interbody devices will initially be available in footprints of 9x24mm, 11x28mm and 11x32mm, with heights of 7 to 14mm, and lordosis of 0 and 6 degrees.
The company will next launch the product with a select group of surgeons and will be building inventory for its full commercial launch.
"Getting adequate graft fill volume and distribution of graft within the intervertebral disc space is one of the most vexing challenges in spinal fusion procedures, which led us to develop the unique flow-thru technology in our KG series products," noted Jeff Kleiner, MD, founder and CEO of Kleiner Device Labs. "The new KG2 Surge system integrates graft delivery with the interbody device delivery. It allows the surgeon to first distract the disc space by inserting the implant, and then packing the entire disc space bilaterally through the KG2 Surge implant, using the attached cannulated inserter. Fully packing the graft with the disc space in the distracted and final position ensures graft/endplate contact. The system's large rectangular cannula allows simplified delivery of a wide range of allograft and autograft products, including viscous and fibrous materials."
The implant's unique I-beam architecture and ramp design allow unrestricted flow to bilaterally fill and integrate the implant into the prepared disc space. The KG2 Surge interbody device is 3-D printed titanium and features a diamond lattice porous structure to facilitate bone ingrowth.
The KG2 Surge flow-thru interbody system comes with the interbody device pre-assembled with the cannula in a single-use sterile tray.
Dr. Kleiner added, "The surgeon opens our tray with a ready-to-use system in their hands. The learning curve is low for operating room staff, and the KG2 Surge system works in both MIS and open approaches. The associated reusable instrument package allows for immediate revision at every step, should the surgeon desire implant repositioning or removal."
KG2 Surge interbody devices will initially be available in footprints of 9x24mm, 11x28mm and 11x32mm, with heights of 7 to 14mm, and lordosis of 0 and 6 degrees.
The company will next launch the product with a select group of surgeons and will be building inventory for its full commercial launch.