Orthopedic Design & Technology Magazine
July/August 2022
-
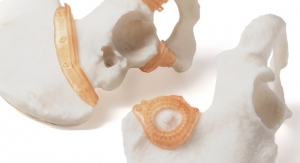
Additive Manufacturing at the Point of Care
The flexibility additive manufacturing offers enables it to be used as a product generation solution at the point-of-care.08.12.22
-

Six Orthopedic Firms En Route to Significant Market Disruption
The ODT Editors have spotlighted six companies outside the Top 10 they feel are making an impact on the device sector.08.12.22
-
 Best PracticesMinding the Gap: How Harmonizing Quality Systems Pays OffFDA issued a proposed rule to harmonize 21 CFR 820 with ISO 13485, which could save medical device companies $439 to $533 million over a decade.08.12.22
Best PracticesMinding the Gap: How Harmonizing Quality Systems Pays OffFDA issued a proposed rule to harmonize 21 CFR 820 with ISO 13485, which could save medical device companies $439 to $533 million over a decade.08.12.22
-
 Process ImprovementFour Key Considerations for OEMs Transferring a Product ProgramTransferring programs can offer a variety of advantages—including with regard to streamlining resources.08.12.22
Process ImprovementFour Key Considerations for OEMs Transferring a Product ProgramTransferring programs can offer a variety of advantages—including with regard to streamlining resources.08.12.22
-
 Regulatory PerspectivesThe Case for Using Historical Control in Clinical Trial DesignHistorical control refers to the use of data from past studies to estimate potential response to a placebo or standard care treatment among trial patients.08.12.22
Regulatory PerspectivesThe Case for Using Historical Control in Clinical Trial DesignHistorical control refers to the use of data from past studies to estimate potential response to a placebo or standard care treatment among trial patients.08.12.22
-
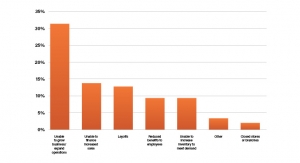 Market SnapshotThe Fundamentals of Go-To-Market Strategy in Orthopedic Medical DevicesGetting the fundamentals right in go-to-market strategy, especially for disruptive products, is critical to success.08.12.22
Market SnapshotThe Fundamentals of Go-To-Market Strategy in Orthopedic Medical DevicesGetting the fundamentals right in go-to-market strategy, especially for disruptive products, is critical to success.08.12.22
-
 The Last WordThe Genetic Risk for Joint Replacement FailureThis could begin a new era where genetic testing prior to receiving medical implants becomes routine.08.12.22
The Last WordThe Genetic Risk for Joint Replacement FailureThis could begin a new era where genetic testing prior to receiving medical implants becomes routine.08.12.22
-
 Top 10 Orthopedic Device FirmsThe ODT 2022 Top 10 Orthopedic Device CompaniesEvery member of the Top 10 posted revenue increases in their latest fiscal years—some even returned to pre-pandemic levels of growth.08.12.22
Top 10 Orthopedic Device FirmsThe ODT 2022 Top 10 Orthopedic Device CompaniesEvery member of the Top 10 posted revenue increases in their latest fiscal years—some even returned to pre-pandemic levels of growth.08.12.22
-
 EditorialOrthopedic Device OEMs Recover Rapidly to Face Supply Chain ChallengesODT's Top Company reports reveal a recovery for orthopedic device makers, but now they face a supply chain crisis.08.12.22
EditorialOrthopedic Device OEMs Recover Rapidly to Face Supply Chain ChallengesODT's Top Company reports reveal a recovery for orthopedic device makers, but now they face a supply chain crisis.08.12.22
-
 ColumnsTrouble Finding Talent? Three People-Centric Measures to Edge Out CompetitorsA growing skills gap is a significant concern for CEOs everywhere. In fact, for the Fortune 500, talent is their biggest worry.08.12.22
ColumnsTrouble Finding Talent? Three People-Centric Measures to Edge Out CompetitorsA growing skills gap is a significant concern for CEOs everywhere. In fact, for the Fortune 500, talent is their biggest worry.08.12.22
-
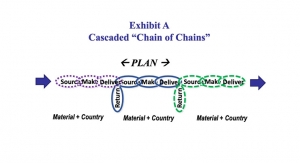 ColumnsThe Interdependence of Supply Chain Stability and World PeaceSupply chains and world peace are codependent—one cannot exist without the other.08.12.22
ColumnsThe Interdependence of Supply Chain Stability and World PeaceSupply chains and world peace are codependent—one cannot exist without the other.08.12.22
















