Business Wire09.22.20
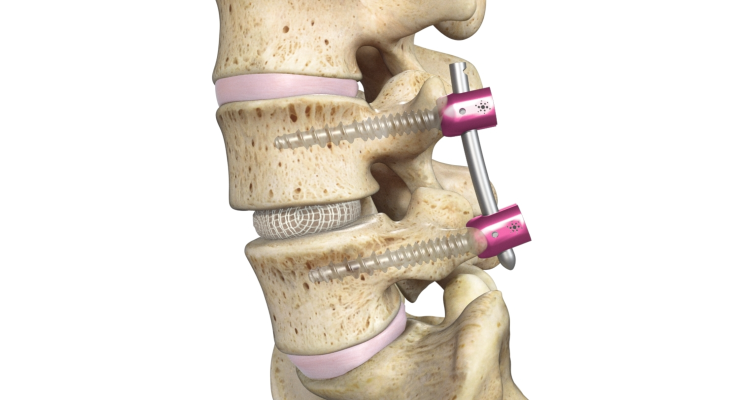
Spineology Inc. has announced the FDA grant of its proprietary Spineology Interbody Fusion System, now called the OptiMesh Expandable Interbody Fusion System. The grant follows the successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption (IDE) trial. OptiMesh is a unique mesh device that expands in three dimensions, enabling surgeons to perform interbody fusion procedures through the smallest access in the spine industry.
The SCOUT IDE trial outcomes data was presented at the Society for Minimally Invasive Spine Surgery (SMISS) Annual Meeting in November 2019, by the trial’s lead investigator, John Chi, M.D., M.P.H. Chi and his co-authors reported:
“We have found this innovative, minimally invasive device to be a safe and effective option for lumbar interbody fusion procedures,” said Chi. “The outcomes, including high fusion rates, improved function, reduced pain and high satisfaction rates, are very favorable. Additionally, the minimal exposure requirements yielded an exceptional safety profile.”
Spineology is preparing for a Q1 2021 launch of the OptiLIF procedure utilizing the OptiMesh device. This procedure enables enhanced recovery and exceptional efficiency, and its outcomes are supported by prospective FDA IDE clinical data.
“OptiLIF is the least invasive lumbar fusion procedure that I can do,” said Dr. Stephane Lavoie of DeLand, Florida, an investigator in the SCOUT study. “The unique OptiMesh implant can be inserted through a one-centimeter incision and then expanded to restore anatomy, which provides neural decompression and optimally conforms to a patient’s endplates. As a result, patients recover quickly, and the impact to procedure efficiency is significant. OptiLIF will have a major impact on the standard of care related to low back and leg pain.”
The FDA De Novo grant of the OptiMesh Expandable Interbody Fusion System opens the door to commercialization of Spineology’s OptiLIF procedure, which supports Spineology’s anatomy-conserving product strategy.
“I am pleased to announce the De Novo grant of our OptiMesh implants and instrumentation to support the OptiLIF procedure. Based on the strong SCOUT study results and experiences of our investigators, we are preparing for a full market launch in Q1 of 2021. I anticipate OptiLIF will help take surgery for low back and leg pain to the next level through its ability to provide excellent patient outcomes, enhanced recovery and exceptional efficiency,” said John Booth, Spineology’s CEO.
The SCOUT IDE trial outcomes data was presented at the Society for Minimally Invasive Spine Surgery (SMISS) Annual Meeting in November 2019, by the trial’s lead investigator, John Chi, M.D., M.P.H. Chi and his co-authors reported:
- Substantial improvements in low back pain and a reduction in functional limitations at six, 12, and 24 months
- A 98 percent fusion rate at 24 months, as seen on CT scans assessed by two independent radiologists
- ~ 90 percent “Excellent” or “Good” patient satisfaction scores at six, 12, and 24 months post-procedure
- An excellent safety record with no serious, device-related, adverse events
“We have found this innovative, minimally invasive device to be a safe and effective option for lumbar interbody fusion procedures,” said Chi. “The outcomes, including high fusion rates, improved function, reduced pain and high satisfaction rates, are very favorable. Additionally, the minimal exposure requirements yielded an exceptional safety profile.”
Spineology is preparing for a Q1 2021 launch of the OptiLIF procedure utilizing the OptiMesh device. This procedure enables enhanced recovery and exceptional efficiency, and its outcomes are supported by prospective FDA IDE clinical data.
“OptiLIF is the least invasive lumbar fusion procedure that I can do,” said Dr. Stephane Lavoie of DeLand, Florida, an investigator in the SCOUT study. “The unique OptiMesh implant can be inserted through a one-centimeter incision and then expanded to restore anatomy, which provides neural decompression and optimally conforms to a patient’s endplates. As a result, patients recover quickly, and the impact to procedure efficiency is significant. OptiLIF will have a major impact on the standard of care related to low back and leg pain.”
The FDA De Novo grant of the OptiMesh Expandable Interbody Fusion System opens the door to commercialization of Spineology’s OptiLIF procedure, which supports Spineology’s anatomy-conserving product strategy.
“I am pleased to announce the De Novo grant of our OptiMesh implants and instrumentation to support the OptiLIF procedure. Based on the strong SCOUT study results and experiences of our investigators, we are preparing for a full market launch in Q1 of 2021. I anticipate OptiLIF will help take surgery for low back and leg pain to the next level through its ability to provide excellent patient outcomes, enhanced recovery and exceptional efficiency,” said John Booth, Spineology’s CEO.