Sean Fenske, Editor-in-Chief09.16.19
The de novo’s regulatory pathway is the third most common method for getting a product through the agency’s device review process, but hardly well understood by most firms. As such, the FDA has given the regulatory route more attention recently to make it more clear for companies seeking to use the option.
Earlier this year, Intellirod Spine announced it had traversed the pathway successfully, enabling the company to bring the industry’s first spinal surgical device to market using the option. Guiding them along the way was MCRA, a clinical research organization and advisory firm with deep industry experience in regulatory, reimbursement, clinical research, healthcare compliance, and quality assurance.
Taking a moment to speak with ODT, Justin Eggleton, VP of Spine Regulatory Affairs at MCRA LLC, explained why the Intellirod Spine story was significant and how it could continue to improve the industry’s understanding of the de novo pathway.
Sean Fenske: Can you tell me what the Intellirod Spine technology is and its therapeutic application area?
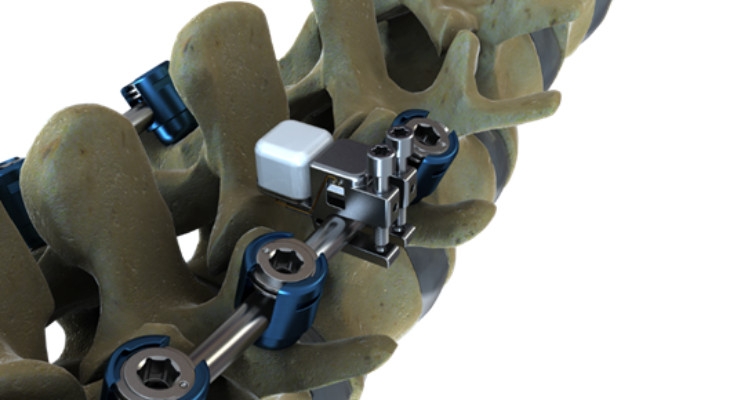
Justin Eggleton: The device is marketed as the LOADPRO Intraoperative Rod Strain Sensor. It is intended for use as an intra-operative tool for adjustment of posterior spine rods and to inform surgeons of rod strain. Historically, surgeons evaluate rod strain simply through tactile feel. This instrument allows surgeons to quantify rod strains and make more informed decisions while contouring the rods and correcting kyphotic deformity during surgery. The observed strains are contextualized by the strain properties of the rod materials, so rods can now be manipulated with a better understanding of rod strain relative to the material yield strain. This could help surgeons learn more about the relationship between rod strain and the common failure modes with these longer constructs.
Fenske: Why is the Intellirod Spine’s de novo decision significant?
Eggleton: It represents the first favorable de novo decision for a device intended to be used in spine surgery. Additionally, it’s a digital health technology directed toward providing more data to the surgeon during the surgical procedure. More importantly, it puts the spotlight on FDA’s more-focused decision making to ensure novel technologies are appropriately regulated based on the level of risk. The de novo pathway used to be poorly understood, which left these novel devices in an unusual regulatory predicament. FDA’s efforts to re-vamp the de novo program really helped industry to define more predictable regulatory pathways for these lower-risk devices that have no predicate devices. It should open the door to more investment in the development of these devices, for which the benefits will trickle down to patients and their quality of life. As digital health technologies continue to improve, it also provides an important pathway that could be a viable option to market.
Fenske: Why haven’t products for the spine used the de novo regulatory pathway in the past?
Eggleton: Most innovation in the spinal space has occurred historically with devices with a known FDA classification: non-fusion devices, additive manufacturing with fusion hardware, surgical instrumentation, etc. I think the lack of innovation for these novel devices is influenced by an unpredictable regulatory pathway. As such, receiving investment capital for a technology with an unpredictable regulatory pathway is not commercially viable. For a disc replacement, we know it will need a clinical study and PMA. There is significant investment, but we know this up front and it can be planned with a return on investment that can justify the resources. For a device such as LOADPRO, receiving funds 10 years ago would be complicated as the regulatory pathway was more unpredictable.
Now that the de novo pathway is better defined, I do think we should begin to see the FDA and industry using it more for these devices where special controls are sufficient to justify Class II designation. It is a viable pathway; however, it is essential for industry to understand the de novo pathway has specific criteria to determine appropriateness; therefore, we will not begin to notice all of these Class III devices for high-risk devices suddenly going through the de novo pathway. In other words, the de novo pathway should not be viewed as a short cut to the market where companies can now navigate around the PMA requirements for Class III devices.
Fenske: What advantages does the de novo pathway offer?
Eggleton: To preface the answer, the de novo pathway is not an option for devices that are Class III requiring a PMA. There is an algorithm to determine if the de novo pathway to Class II is appropriate for a novel device. It starts with lack of a predicate device, different intended use, or different technological features with different safety and effectiveness questions. From there, you need to be able to describe the risks and benefits to demonstrate that general and special controls are sufficient to properly regulate the device as Class II. Once the de novo is granted, it is automatically put into Class II and industry can cite it as a predicate device for their 510(k).
The requirements for future 510(k)s that all cite a de novo will vary device to device. It’s not going to be clear if the first few will also require clinical data or if the special controls can be accommodated through non-clinical testing alone. So I recommend initiating a dialogue with FDA early through a pre-submission to avoid unnecessarily driving product development in the wrong direction.
De novo submissions all require clinical data specific to the subject device. A clinical foundation is necessary in order to develop special controls. The types of devices vary, and so does the type of clinical data needed. I position this as an advantage since these devices will have clinical data available upon granting of the de novo, which benefits marketing and reimbursement activities. Even if the downstream 510(k)s that claim equivalence do not require clinical data, they may face marketing barriers due to the lack of clinical data.
Fenske: Can you speak to the marketing aspects further? What are the marketing benefits to this product being the first spinal device to gain a de novo?
Eggleton: The marketing benefit is driven by the press coverage that raises awareness of the positive de novo decision granting Class II designation. Surgeons will be attracted to the technology when they study the benefits of a new instrument that allows more repeatable and clinically meaningful decisions about rod contouring to be quantified and recorded. While there is a lot we can learn from these values compared to the material’s mechanical properties, I believe the clinical benefit will be more defined over time if the community adopts these measurement techniques to posterior spine surgery. I expect this dynamic will continue to trend in this direction as surgeons are always seeking opportunities to improve the treatments they offer their patients.
Fenske: Do you think this decision will have any effect on the use of the de novo pathway for future spine products?
Eggleton: I think the work required to get to this decision will positively influence FDA’s use of the de novo pathway for future spine devices. While it’s nice to hang our hat on the positive decision, all parties involved worked really well together to ensure the risks were well understood and weighed against the benefits. This contributed to labeling discussions with FDA that benefited from the time spent understanding the device’s technological characteristics and performance data. So I think the “lessons learned” from the collaboration will certainly be a positive influence to future de novo submissions for spine devices. This applies to the FDA review and working with MCRA to support navigating the pathway.
Fenske: What are the next steps for the Intellirod Spine device?
Eggleton: Intellirod Spine can provide these details. However, their next steps are in the public domain. This includes an implantable version of LOADPRO (ACCUVISTA) that would allow rod strains to be measured post-operatively. There are several potential benefits to these data that will be assessed in future performance studies. They also have an interesting wearable sensor to measure brace compliance. While there are clinical benefits to ensuring compliance, I imagine there is interest from the insurance/reimbursement community.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Eggleton: I always recommend early interaction with FDA. I think industry and FDA benefit from a collaborative process where both sides are involved in device development. This sentiment is echoed by FDA’s commitment to the Total Product Life Cycle. Today’s FDA has learned that devices are not designed in a silo and are constantly being improved. With the speed at which information is available to the public, FDA has learned that it benefits from being involved early and often. Often times, I hear industry doesn’t want to submit early due to fear of the FDA response. FDA will have the same response whether you find out now in 2019 or after you’ve spent the money and time to complete performance evaluations in 2021. Intellirod Spine is a great example of a company who interacted with FDA early and often. The feedback was not always positive, but with both sides working together, we were granted a positive de novo decision. Obviously, the LOADPRO Total Product Life Cycle will benefit from the history established with FDA.
Earlier this year, Intellirod Spine announced it had traversed the pathway successfully, enabling the company to bring the industry’s first spinal surgical device to market using the option. Guiding them along the way was MCRA, a clinical research organization and advisory firm with deep industry experience in regulatory, reimbursement, clinical research, healthcare compliance, and quality assurance.
Taking a moment to speak with ODT, Justin Eggleton, VP of Spine Regulatory Affairs at MCRA LLC, explained why the Intellirod Spine story was significant and how it could continue to improve the industry’s understanding of the de novo pathway.
Sean Fenske: Can you tell me what the Intellirod Spine technology is and its therapeutic application area?
Justin Eggleton: The device is marketed as the LOADPRO Intraoperative Rod Strain Sensor. It is intended for use as an intra-operative tool for adjustment of posterior spine rods and to inform surgeons of rod strain. Historically, surgeons evaluate rod strain simply through tactile feel. This instrument allows surgeons to quantify rod strains and make more informed decisions while contouring the rods and correcting kyphotic deformity during surgery. The observed strains are contextualized by the strain properties of the rod materials, so rods can now be manipulated with a better understanding of rod strain relative to the material yield strain. This could help surgeons learn more about the relationship between rod strain and the common failure modes with these longer constructs.
Fenske: Why is the Intellirod Spine’s de novo decision significant?
Eggleton: It represents the first favorable de novo decision for a device intended to be used in spine surgery. Additionally, it’s a digital health technology directed toward providing more data to the surgeon during the surgical procedure. More importantly, it puts the spotlight on FDA’s more-focused decision making to ensure novel technologies are appropriately regulated based on the level of risk. The de novo pathway used to be poorly understood, which left these novel devices in an unusual regulatory predicament. FDA’s efforts to re-vamp the de novo program really helped industry to define more predictable regulatory pathways for these lower-risk devices that have no predicate devices. It should open the door to more investment in the development of these devices, for which the benefits will trickle down to patients and their quality of life. As digital health technologies continue to improve, it also provides an important pathway that could be a viable option to market.
Fenske: Why haven’t products for the spine used the de novo regulatory pathway in the past?
Eggleton: Most innovation in the spinal space has occurred historically with devices with a known FDA classification: non-fusion devices, additive manufacturing with fusion hardware, surgical instrumentation, etc. I think the lack of innovation for these novel devices is influenced by an unpredictable regulatory pathway. As such, receiving investment capital for a technology with an unpredictable regulatory pathway is not commercially viable. For a disc replacement, we know it will need a clinical study and PMA. There is significant investment, but we know this up front and it can be planned with a return on investment that can justify the resources. For a device such as LOADPRO, receiving funds 10 years ago would be complicated as the regulatory pathway was more unpredictable.
Now that the de novo pathway is better defined, I do think we should begin to see the FDA and industry using it more for these devices where special controls are sufficient to justify Class II designation. It is a viable pathway; however, it is essential for industry to understand the de novo pathway has specific criteria to determine appropriateness; therefore, we will not begin to notice all of these Class III devices for high-risk devices suddenly going through the de novo pathway. In other words, the de novo pathway should not be viewed as a short cut to the market where companies can now navigate around the PMA requirements for Class III devices.
Fenske: What advantages does the de novo pathway offer?
Eggleton: To preface the answer, the de novo pathway is not an option for devices that are Class III requiring a PMA. There is an algorithm to determine if the de novo pathway to Class II is appropriate for a novel device. It starts with lack of a predicate device, different intended use, or different technological features with different safety and effectiveness questions. From there, you need to be able to describe the risks and benefits to demonstrate that general and special controls are sufficient to properly regulate the device as Class II. Once the de novo is granted, it is automatically put into Class II and industry can cite it as a predicate device for their 510(k).
The requirements for future 510(k)s that all cite a de novo will vary device to device. It’s not going to be clear if the first few will also require clinical data or if the special controls can be accommodated through non-clinical testing alone. So I recommend initiating a dialogue with FDA early through a pre-submission to avoid unnecessarily driving product development in the wrong direction.
De novo submissions all require clinical data specific to the subject device. A clinical foundation is necessary in order to develop special controls. The types of devices vary, and so does the type of clinical data needed. I position this as an advantage since these devices will have clinical data available upon granting of the de novo, which benefits marketing and reimbursement activities. Even if the downstream 510(k)s that claim equivalence do not require clinical data, they may face marketing barriers due to the lack of clinical data.
Fenske: Can you speak to the marketing aspects further? What are the marketing benefits to this product being the first spinal device to gain a de novo?
Eggleton: The marketing benefit is driven by the press coverage that raises awareness of the positive de novo decision granting Class II designation. Surgeons will be attracted to the technology when they study the benefits of a new instrument that allows more repeatable and clinically meaningful decisions about rod contouring to be quantified and recorded. While there is a lot we can learn from these values compared to the material’s mechanical properties, I believe the clinical benefit will be more defined over time if the community adopts these measurement techniques to posterior spine surgery. I expect this dynamic will continue to trend in this direction as surgeons are always seeking opportunities to improve the treatments they offer their patients.
Fenske: Do you think this decision will have any effect on the use of the de novo pathway for future spine products?
Eggleton: I think the work required to get to this decision will positively influence FDA’s use of the de novo pathway for future spine devices. While it’s nice to hang our hat on the positive decision, all parties involved worked really well together to ensure the risks were well understood and weighed against the benefits. This contributed to labeling discussions with FDA that benefited from the time spent understanding the device’s technological characteristics and performance data. So I think the “lessons learned” from the collaboration will certainly be a positive influence to future de novo submissions for spine devices. This applies to the FDA review and working with MCRA to support navigating the pathway.
Fenske: What are the next steps for the Intellirod Spine device?
Eggleton: Intellirod Spine can provide these details. However, their next steps are in the public domain. This includes an implantable version of LOADPRO (ACCUVISTA) that would allow rod strains to be measured post-operatively. There are several potential benefits to these data that will be assessed in future performance studies. They also have an interesting wearable sensor to measure brace compliance. While there are clinical benefits to ensuring compliance, I imagine there is interest from the insurance/reimbursement community.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Eggleton: I always recommend early interaction with FDA. I think industry and FDA benefit from a collaborative process where both sides are involved in device development. This sentiment is echoed by FDA’s commitment to the Total Product Life Cycle. Today’s FDA has learned that devices are not designed in a silo and are constantly being improved. With the speed at which information is available to the public, FDA has learned that it benefits from being involved early and often. Often times, I hear industry doesn’t want to submit early due to fear of the FDA response. FDA will have the same response whether you find out now in 2019 or after you’ve spent the money and time to complete performance evaluations in 2021. Intellirod Spine is a great example of a company who interacted with FDA early and often. The feedback was not always positive, but with both sides working together, we were granted a positive de novo decision. Obviously, the LOADPRO Total Product Life Cycle will benefit from the history established with FDA.