Sam Brusco, Associate Editor03.29.19
The next significant casualty of the deadly opioid crisis may in fact be a pharmaceutical manufacturer.
Purdue Pharma, the maker of OxyContin (the brand name for the opioid analgesic oxycodone hydrochloride) is reportedly exploring filing for bankruptcy to tackle the liabilities from about 2,000 lawsuits. All of these actions allege the drugmaker and its wealthy owners, the Sackler family, contributed to the crisis by misleading doctors and patients about the risks of prolonged prescription opioid use. Purdue denied the allegations, and according to The Guardian, claimed the FDA-approved labels for its opioids displayed warnings concerning the risk of abuse and misuse during pain treatments.
The company faces a May trial brought by Oklahoma’s attorney general that also accuses Purdue of contributing to a surge of fatal overdoses by flooding the market with the highly addictive drugs and falsely proclaiming their safety. The drugmaker sought to dispel the bankruptcy rumors in a Feb. 22 court filing for this case. “Purdue is still here—ready, willing, and eager to prove in this court that the State’s claims are baseless,” the company said in court papers.
The Oklahoma case and other similar lawsuits seek damages from Purdue and other drugmakers accused of fueling the opioid crisis. In addition to over 1,600 lawsuits consolidated in the Ohio federal court, more than 300 cases are pending in state courts, and dozens of state attorneys general have sued manufacturers, Purdue included.
President Donald Trump has been outspoken about the opioid crisis. In October 2017, he declared it a public health emergency. Last March, he unveiled the Initiative to Stop Opioids Abuse and Reduce Drug Supply and Demand to confront the driving forces behind the crisis. The initiative aims to attack the problem on three fronts. Americans will be educated about the dangers of opioid and other drug use. The initiative will also attempt to crack down on international and domestic illicit drug supply chains devastating American communities. Finally, those struggling with addiction will be helped through evidence-based treatment and recovery support services.
When announcing all of this, President Trump also addressed the pharmaceutical companies’ fault in the crisis. “Companies must also be held accountable,” he said in a statement last March at Manchester Community College in Manchester, N.H. “The Department of Justice recently created a task force to coordinate investigations and lawsuits against manufacturers and other bad actors that harm our citizens.”
One of the goals for the education arm of President Trump’s Opioid Initiative is supporting research and development efforts for innovative technologies and additional therapies to prevent addiction and decrease opioid use. In May of last year, two months after President Trump’s announcement, the FDA launched an innovation challenge supporting this initiative. The FDA’s challenge is intended to spur development of medical devices, diagnostic tests, and digital health technologies (mobile health applications included) to help combat the opioid crisis and achieve prevention and treatment of opioid use disorder (OUD).
Officially titled, “FDA Innovation Challenge: Devices to Prevent and Treat Opioid Use Disorder,” the agency received overwhelming response from the medtech community. Over 250 applications were received from medtech companies large and small. After careful review, the FDA chose eight challenge participants. Their devices provided solutions attacking all fronts—devices from the selected participants included those that predict OUD risk, spot an overdose, dispense medication, and provide pain treatment alternatives.
“In late 2018, we were selected as one of eight companies worldwide to participate in the innovation challenge to tackle the opioid epidemic,” said John Tushar, president of global franchises for Avanos Medical, an Alpharetta, Ga.-based medical device company focused on delivering breakthrough solutions that will help patients get back to the things that matter. Avanos is committed to creating the next generation of healthcare solutions that address reducing the use of opioids while helping patients move from surgery to recovery. “As a winner, we are working closely with the FDA to get our enhanced nerve visualization system cleared for commercialization. Our selection further supports the company’s initiative to provide innovative non-opioid therapies to eliminate or reduce the use of opioids for surgical patients.”
Similar to what occurs during the FDA’s Breakthrough Devices Program, the applicants will work directly with the agency to accelerate development and expedite marketing application review for their products. Breakthrough Device designation will be awarded to each device meeting the statutory criteria for designation without submitting a separate application.
Under the leadership of departing Commissioner Scott Gottlieb, the FDA has also taken several other steps to combat the crisis. The agency has fortified its enforcement and interdiction work targeting illegal, unapproved, counterfeit, and potentially dangerous drugs shipped illegally through international mail facilities (IMFs). The FDA also has special agents assigned to the IMFs who work with U.S. Customs and Border Protection and FDA regulatory staff to identify and refer suspect shipments. They then bring each case to prosecution together with the U.S. Department of Justice.
In mid-February, the FDA issued its first warning letter under the Drug Supply Chain Security Act (DSCSA) to McKesson Corp. over concerns about tampering with opioid medications. Under the DSCSA, manufacturers, repackagers, wholesale distributors, and dispensers—which are mainly pharmacies—are all required to have systems and processes in place to quarantine and investigate suspect and illegitimate medications.
This year, the agency intends publish new guidance describing its application of the risk/benefit framework for opioid analgesics in order to consider the full range of risks associated with the drugs, including risks created by illicit use. The FDA also plans to solicit input from the public to understand how it should evaluate new opioid drugs relative to those already existing on the market. New guidance will also be developed to modernize the development pathway for non-opioid analgesics to treat acute or chronic pain. As part of this push, the FDA will identify specific clinical conditions—like post-surgical pain or back pain—to speed development of new painkillers seeking narrow indications.
Also in mid-February, U.S. Sen. Patty Murray (D-WA), a ranking member of the U.S. Senate Health, Education, Labor, and Pensions (HELP) Committee, delivered highly evocative remarks at the Committee’s hearing on pain management during the opioid crisis. Murray emphasized the necessity of ensuring pain patients’ access to pain treatment while guaranteeing opioids are marketed, prescribed, and used responsibly. She also stressed that providers should be helping patients access the pain management tools best meeting their needs and called for further action to build on the steps taken last year to address the crisis.
“50 million people nationwide suffer from pain that persists for weeks or years,” Murray said in her opening remarks. “For almost 20 million people, this pain can interfere with their work and daily life activities. Pain management is an absolutely critical quality of life issue for these people and their families. Acute and chronic pain can make it harder to keep a job and earn a paycheck. Even when treated, pain can make it difficult to travel to and from work and sit at a desk for long stretches of time. And pain doesn’t just affect a person’s livelihood—it affects every aspect of their life. Without pain management, patients may not be able to tackle the tasks they need to live independently, like getting dressed, driving a car, or doing laundry. They may not be able to spend quality time with loved ones, as their pain can make it hard to enjoy a meal with friends and family, attend a grandkid’s soccer game, or even leave the house. Without the right pain management tools, some patients struggle to get a decent night’s sleep.”
Medical technologies are an integral set of tools for an effective pain management regimen that seeks to reduce or remove the number of opioid drugs involved in the treatment. These technologies range from a simple heating pad to alleviate muscle pain to targeted drug delivery devices for post-surgical recovery to a surgical or implantable neurostimulation strategy that restricts pain signals to the brain (often replacing them with paresthesia, a tingling sensation).
“Millions of Americans wake up every day suffering from chronic or acute pain, and it is critical that they have access to non-opioid treatments to help them lead productive lives,” Mark Leahey, president and CEO of the Medical Device Manufacturers Association (MDMA) commented in response to the Senate HELP Committee hearing. “Medical technologies provide life-changing relief for many who are suffering in pain, but these cures and therapies do no good if patients and physicians cannot access them. MDMA looks forward to working with Congress and all policy makers to improve access to and awareness of medical technologies so the full potential of this industry can be realized as we all work towards our common goal of ending the opioid crisis.”
As part of the efforts to address the opioid epidemic, device-delivered therapies are being considered as alternatives or adjunct to systemic opioids. One major benefit patients may find using a technology to manage pain is a greater feeling of control over their pain. Chronic pain patients not fully aware of technological alternatives may feel like they are a slave to their pills. However, the bullish strategy the White House, Congress, and the FDA are taking to mitigate this crisis may persuade patients and healthcare practitioners in a different way—patients and physicians will hopefully pursue alternative methods because the public opinion of opioid analgesics has plummeted over the past few years.
“100 million Americans have chronic pain,” explained Shai Gozani M.D., Ph.D., chairman, president, and CEO of NeuroMetrix, a Waltham, Mass.-based bioelectrical and digital medicine company driving innovation to address chronic health conditions including chronic pain and diabetes. “Many are prescribed opioids despite side effects and the potential for addiction, which has contributed to the devastating opioid crisis.
There’s a desperate need for non-pharmacological pain treatments. Recent research shows people living with chronic pain feel stigmatized by the opioid crisis and are looking for alternative treatments.1”
For far too long, opioid therapy was considered a “one-size-fits-all” solution to managing any sort of pain, acute or chronic. The drugs worked and continued to work as intended, so no alarm was raised for patients using it in a long-term setting. Managing pain is a complex task, mainly because the therapy depends on the underlying condition. Unfortunately, patients may use painkillers without understanding the complexity of their pain and the condition responsible for it. Using an opioid drug to treat a specific source of pain is like using only an air freshener to cover up a bad stench—the bad smell might be replaced with a more pleasant fragrance, but it’s going to waft right back to the nostrils unless the source of that smell is cleaned up.
Using technology in place of drugs also puts pain control in the patient’s hands. Using a drug is not something a patient actively participates in—the drug must be taken at specific intervals, and there is always the looming threat of the pain returning once the pharmaceutical has worn off. The dependence on these medicines becomes more than physical because of this mindset. Without the drug, pain patients can feel powerless to fight their condition. When using a technological solution, the patient can choose when and at what intensity to apply a therapy once their pain level becomes too high. That level of control over a previously debilitating condition can prove to be empowering for a patient who previously felt like a slave to painkillers.
“There is no one-size-fits-all approach to pain management and each person experiences pain differently,” declared Tushar. “While opioid prescriptions have long been a viable treatment for a variety of pain conditions, they have led to the drug addiction epidemic and new ways to successfully and safely treat pain are necessary for today’s patients. Innovative solutions to pain management such as device alternatives can help patients optimize their pain control to best meet their needs and improve care, while simultaneously reducing the need for opioids.”
Neurostimulation technology (neurostim for short) has been a promising avenue for non-pharmacological pain management, and a source of innovation for companies seeking to enter the newly burgeoning market. First approved by the FDA in 1989, neurostim uses modern technology and electrical pulses to manage chronic pain due to damaged or improperly functioning nerves. Neurostim devices overcome the abnormal sensations these nerves produce with a different electrical stimulus more pleasant than the original sensation—patients report feeling a tingling sensation. The technique involves implantation of a small device into the thoracic spine (for lower back or leg pain) or the neck (for upper back and arm pain). The patient essentially controls the pain with a handheld programmer.
Depending on where the electrical stimulus is applied in the body, it is either referred to as spinal cord stimulation (SCS) or peripheral nerve stimulation (PNS), both a form of neuromodulation. SCS therapy has been used to treat conditions like complex regional pain syndrome (CRPS) or failed-back surgery syndrome. According to the International Neuromodulation Society, SCS therapy now accounts for about 70 percent of all neuromodulation treatments. That number is predicted to rise to manage a variety of chronic long-term conditions.
PNS therapy is also commonly used to treat chronic pain. It involves surgery to implant a small electrical device next to one of the peripheral nerves—those located beyond the brain or spinal cord. Interestingly enough, PNS was invented in the mid-1960s, even before the commonly used SCS. Since then, it has become established for a number of clinical conditions including certain types of CRPS and pain due to peripheral nerve injuries. Some common PNS applications also include back pain treatment, occipital nerve stimulation to treat migraines, and pudendal nerve stimulation that is being investigated for urinary bladder incontinence.
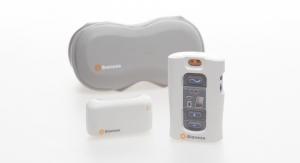
“Medical technology is an important part of a pain management regimen because the technology, specific to neuromodulation, has progressed to provide effective, safe, non-addictive, reversible, and long-term solutions for chronic pain treatment,” said Mark Geiger, global director of marketing, implantables, for Bioness Inc., a Valencia, Calif.-based medical device company that provides technologies helping people to regain mobility and independence. The company produces implantable and external neuromodulation systems, robotic systems, and software-based therapy programs providing functional and therapeutic benefits for those affected by pain, central nervous system disorders, and orthopedic injuries. “Many neuromodulation devices, including PNS products like the StimRouter, are minimally invasive and only require a 15-30-minute implant by a doctor while the patient is awake under local anesthesia. These devices can put the patient in charge of their own pain management at home.”
Bioness’ StimRouter first entered the U.S. market in 2015 following FDA clearance. StimRouter uses PNS therapy to treat intractable pain originating in a peripheral nerve. Applications for the technology include chronic pain conditions located at or related to the upper and lower limbs, entrapment syndromes, intercostal neuralgias, or other peripheral injuries or diseases. The StimRouter implant consists of a passive, 15 cm lead with electrodes at one end and a receiver at the other that is implanted percutaneously with a small incision under local anesthesia. The lead’s tip is placed near the peripheral nerve causing the chronic pain.
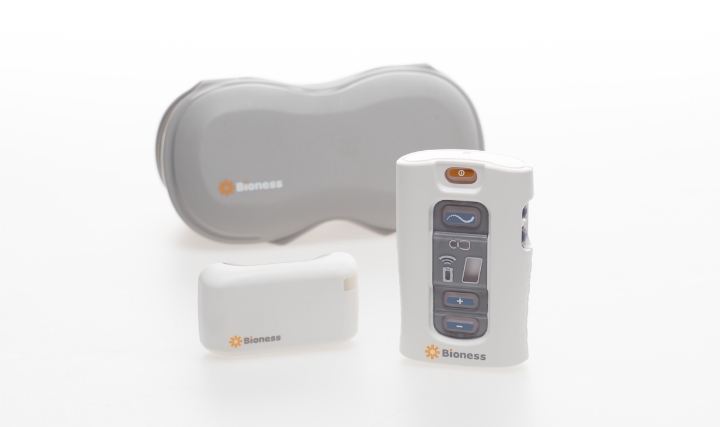
StimRouter is the only implantable neuromodulation device currently marketed for adults with chronic pain of peripheral nerve origin. Image courtesy of Bioness.
An External Pulse Transmitter “patch” is worn on the skin over the implant, powering it and sending the stimulation signal to the implant. The patient controls their desired stimulation with a “Patient Programmer” resembling a small remote control. The programmer turns the system on, off, up, and down to control the pain, and is equipped with a display to monitor and manage stimulation programs and the level of stimulation intensity.
“The StimRouter was the first system of its kind designed specifically to treat focal, chronic pain of a peripheral nerve origin,” said Geiger. “When compared to the more established spinal cord stimulation systems offered by several large medical device corporations (which involves major surgery with a large, global implant), the StimRouter has the electronics (processor, battery) residing on the wearable outside the body, resulting in a very small, simple implant process while the patient is awake. This eliminates many of the risks typically associated with a spinal cord stimulator, such as implant migration.”
According to the Rheumatoid Arthritis Support Network, as of 2018 rheumatoid arthritis (RA) affected over 1.3 million Americans and as much as 1 percent of the entire world population. The autoimmune disease mainly attacks the synovial tissues within the joints. RA can affect the entire body, and can attack organs like the heart, lungs, or tissues like muscles, cartilage, and ligaments. RA causes chronic swelling and pain that can be severe, and can cause permanent disability.
The pain experienced by RA patients manifests differently than neuropathic pain, requiring different treatment options as a result. Typically, biologic therapies are used to address the underlying chronic inflammation, which may reduce patients’ pain. Unfortunately, biologic therapies can suppress the immune system and aren’t always effective, leading to refractory disease. There is an enormous unmet need for new treatments that are more effective, less immunosuppressive, and more cost-effective than current therapies as a result.
“Despite significant progress with pharmacological therapy, 30-50 percent of RA patients either don’t adequately respond to or are intolerant of biologic therapies,” explained Murthy Simhambhatla, Ph.D., CEO of SetPoint Medical, a Valencia, Calif.-based bioelectronic medicine company dedicated to treating patients with chronic autoimmune diseases. “These therapies can have serious side effects and impose a significant cost burden on the healthcare system. In fact, some of the highest revenue-grossing drugs in history are indicated for patients with RA and other inflammatory autoimmune diseases, and these drugs come with FDA black box warnings regarding serious side effects. Furthermore, patient compliance is a major issue as these drugs typically are injectable therapies.”
Bioelectronic medicine (i.e., nerve stimulating or nerve blocking technology) is a fairly new approach to treat disease and injury. When implanted on a nerve or held against the skin, bioelectronic medical devices can potentially modulate specific nerve activity, elicit a specific change in organ function, and restore health without the complicated side effects of pharmaceuticals.
“We are pioneering the use of bioelectric medicine to fundamentally alter the treatment paradigm for autoimmune diseases,” said Simhambhatla. “Bioelectronic medicine activates a natural physiologic pathway through vagus nerve stimulation to restore immunological homeostasis—or, return the body to its natural ‘set point.’”
The company’s bioelectronic medicine platform delivers targeted electronic pulses to trigger the intrinsic inflammatory reflex. That pathway has found promise as a treatment option for RA, Crohn’s disease, and other chronic autoimmune diseases. SetPoint’s bioelectronic platform consists of a small implantable MicroRegulator, a wireless charging collar or band, and an iPad-based prescription application.
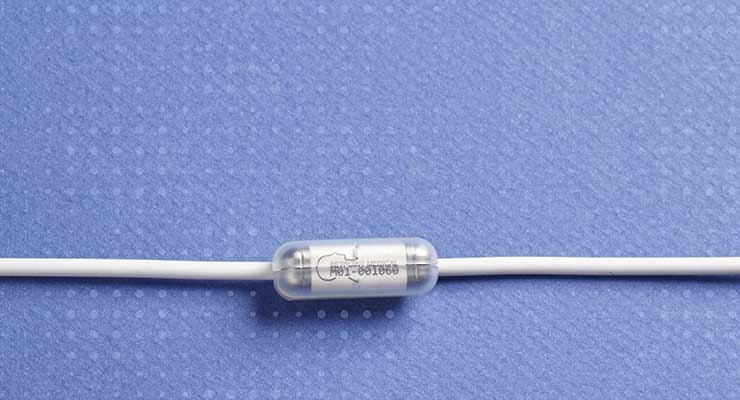
SetPoint Medical's miniature bioelectronic medicine device delivers electrical doses designed to activate the innate inflammatory reflex to produce a systemic anti-inflammatory effect and help restore balance and regulate the immune system. Image courtesy of SetPoint Medical.
“SetPoint’s miniature (less than one inch long) bioelectronic medicine device is implanted on the left vagus nerve via a small incision in the neck. It delivers electrical impulses designed to activate the innate inflammatory reflex to produce a systemic anti-inflammatory effect, and to help restore balance and regulate the immune system.”
The company recently completed its U.S. Pilot IDE Study in patients with multi-drug refractory RA. The study was successful in further validating the device’s mechanism, indicating that it was safe and well-tolerated. According to Simhambhatla, SetPoint anticipates initiating a larger clinical study to evaluate the bioelectronic platform’s safety and efficacy in RA patients in the second half of this year.
Transcutaneous electrical nerve stimulation (TENS) therapy use low-voltage electric currents to treat pain. Electrodes or mediums for electricity to travel to the body are placed on the site of pain, and the currents block pain receptors from being sent from the nerves to the brain. Usually the patient will receive a small, battery-operated TENS machine to use at home. TENS therapy can be used to treat both chronic and acute pain, and TENS is most commonly used to treat osteoporosis- or fibromyalgia-related joint, bone, or muscle problems, tendinitis, bursitis, and neck, labor, and cancer pain.
NeuroMetrix’s Quell, a wearable, over-the-counter (OTC) TENS device, was announced at the 2015 Consumer Electronics Show (CES). The device is worn on the leg and sends neural pulses to the brain that trigger a natural pain relief response in the central nervous system. According to the company, since first launching Quell, almost 200,000 individuals living with chronic pain have experienced its patented neurotechnology.

Wearable technology, through miniaturization, automation, and user-centric design allows a person living with chronic pain to receive treatment and experience relief whenever they need it. Image courtesy of NeuroMetrix.
Quell 2.0, the next-generation iteration of the wearable TENS device, was launched at this year’s CES. Quell 2.0 is about 50 percent smaller than the original product, but features a larger battery to provide more therapy sessions per charge. It is powered by the Quell Relief App, which lets users calibrate the device, start and stop therapy, adjust intensity, and track sleep, activity, pain, and gait. It enables customized therapy dosages, sleep modes, and can adapt to changes in local weather, providing user alerts and the option to increase dosage if the weather is predicted to change in a manner that affects the user’s pain.
“Quell is a more advanced way to treat chronic pain,” said Dr. Gozani. “Like TENS units and implantable spinal stimulators, Quell uses electrical nerve stimulation to provide pain relief. Quell 2.0 is up to 10x more powerful than typical OTC TENS devices.2 Quell is also the only OTC electrical nerve stimulation device FDA cleared for use while sleeping and can be used during the day when active or at night.”

Chronic pain affects many people 24/7, making it important for a technolgy solution to fit seamlessly into their life. Image courtesy of NeuroMetrix.
The company is looking to bring even more personalization to Quell in the future, and expects to upgrade Quell 2.0’s firmware later this year. The upgrade will allow the technology to deliver on the promise of artificial intelligence. The algorithms will be derived from the application of machine learning to millions of data points from over 70,000 chronic pain sufferers in the company’s Quell Health Cloud.
“The process evaluates demographics, health conditions, pain levels and characteristics, device utilization, and objective measures of sleep, activity, and gait from Quell users to power truly personalized treatment,” explained Dr. Gozani. “Quell is already smart enough to adjust a user’s therapy based on manual intensity changes, body position, and sleep movements. With this upgrade, setup and use will be even more precise.”
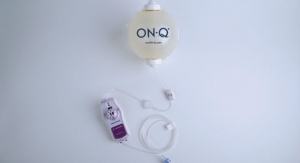
Avanos Medical’s ON-Q* Pain Relief System aims to reduce the number of opioids used for post-surgical recovery. The opioid-sparing elastomeric ambulatory infusion pump automatically and continuously delivers a regulated flow of local anesthetic to a surgical site or in close proximity to nerves, providing targeted pain relief for up to five days. It also features a variable rate controller that changes flow rate according to patients’ individual pain relief requirements.
“Patients treated with ON-Q* may experience faster recovery and less post-operative pain with fewer side effects than patients taking opioids alone,” said Tushar. “ON-Q* may help surgical patients to avoid taking that first opioid, which could otherwise lead to an addiction.”
Avanos’ COOLIEF* Cooled Radiofrequency (RF) is a thermal RF system that uses water-cooled technology (hence the name) to deactivate pain-causing sensory nerves. It works via a RF generator that transmits a small current of energy through an insulated electrode placed within tissue. The friction of charged molecules produces ionic heating, which thermally deactivates the offending nerves. According to the company, delivering RF energy through water-cooled electrodes allows more energy to be safely delivered to target nerves and create ideal spherically-shaped lesions. The system can currently be used to treat chronic sacroiliac joint pain, symptomatic discogenic pain, lumbar medial branch neurotomy, chronic thoracic facet pain, and cervical pain.
Last year, the company acquired Game Ready, a cold and intermittent compression therapy system used for injury and recovery from orthopedic surgery. The system delivers targeted cold and compression therapy through anatomically-designed wraps to help reduce pain and swelling, and reduce opioid consumption.
“Adding this market-leading technology to our portfolio of non-opioid treatment options further commits the company to the innovative fight to reduce the use of opioids,” declared Tushar.
Fight on.
References
More information about opioid-sparing pain management and the technologies featured in this article is available via the interviews below:
Purdue Pharma, the maker of OxyContin (the brand name for the opioid analgesic oxycodone hydrochloride) is reportedly exploring filing for bankruptcy to tackle the liabilities from about 2,000 lawsuits. All of these actions allege the drugmaker and its wealthy owners, the Sackler family, contributed to the crisis by misleading doctors and patients about the risks of prolonged prescription opioid use. Purdue denied the allegations, and according to The Guardian, claimed the FDA-approved labels for its opioids displayed warnings concerning the risk of abuse and misuse during pain treatments.
The company faces a May trial brought by Oklahoma’s attorney general that also accuses Purdue of contributing to a surge of fatal overdoses by flooding the market with the highly addictive drugs and falsely proclaiming their safety. The drugmaker sought to dispel the bankruptcy rumors in a Feb. 22 court filing for this case. “Purdue is still here—ready, willing, and eager to prove in this court that the State’s claims are baseless,” the company said in court papers.
The Oklahoma case and other similar lawsuits seek damages from Purdue and other drugmakers accused of fueling the opioid crisis. In addition to over 1,600 lawsuits consolidated in the Ohio federal court, more than 300 cases are pending in state courts, and dozens of state attorneys general have sued manufacturers, Purdue included.
President Donald Trump has been outspoken about the opioid crisis. In October 2017, he declared it a public health emergency. Last March, he unveiled the Initiative to Stop Opioids Abuse and Reduce Drug Supply and Demand to confront the driving forces behind the crisis. The initiative aims to attack the problem on three fronts. Americans will be educated about the dangers of opioid and other drug use. The initiative will also attempt to crack down on international and domestic illicit drug supply chains devastating American communities. Finally, those struggling with addiction will be helped through evidence-based treatment and recovery support services.
When announcing all of this, President Trump also addressed the pharmaceutical companies’ fault in the crisis. “Companies must also be held accountable,” he said in a statement last March at Manchester Community College in Manchester, N.H. “The Department of Justice recently created a task force to coordinate investigations and lawsuits against manufacturers and other bad actors that harm our citizens.”
One of the goals for the education arm of President Trump’s Opioid Initiative is supporting research and development efforts for innovative technologies and additional therapies to prevent addiction and decrease opioid use. In May of last year, two months after President Trump’s announcement, the FDA launched an innovation challenge supporting this initiative. The FDA’s challenge is intended to spur development of medical devices, diagnostic tests, and digital health technologies (mobile health applications included) to help combat the opioid crisis and achieve prevention and treatment of opioid use disorder (OUD).
Officially titled, “FDA Innovation Challenge: Devices to Prevent and Treat Opioid Use Disorder,” the agency received overwhelming response from the medtech community. Over 250 applications were received from medtech companies large and small. After careful review, the FDA chose eight challenge participants. Their devices provided solutions attacking all fronts—devices from the selected participants included those that predict OUD risk, spot an overdose, dispense medication, and provide pain treatment alternatives.
“In late 2018, we were selected as one of eight companies worldwide to participate in the innovation challenge to tackle the opioid epidemic,” said John Tushar, president of global franchises for Avanos Medical, an Alpharetta, Ga.-based medical device company focused on delivering breakthrough solutions that will help patients get back to the things that matter. Avanos is committed to creating the next generation of healthcare solutions that address reducing the use of opioids while helping patients move from surgery to recovery. “As a winner, we are working closely with the FDA to get our enhanced nerve visualization system cleared for commercialization. Our selection further supports the company’s initiative to provide innovative non-opioid therapies to eliminate or reduce the use of opioids for surgical patients.”
Similar to what occurs during the FDA’s Breakthrough Devices Program, the applicants will work directly with the agency to accelerate development and expedite marketing application review for their products. Breakthrough Device designation will be awarded to each device meeting the statutory criteria for designation without submitting a separate application.
Under the leadership of departing Commissioner Scott Gottlieb, the FDA has also taken several other steps to combat the crisis. The agency has fortified its enforcement and interdiction work targeting illegal, unapproved, counterfeit, and potentially dangerous drugs shipped illegally through international mail facilities (IMFs). The FDA also has special agents assigned to the IMFs who work with U.S. Customs and Border Protection and FDA regulatory staff to identify and refer suspect shipments. They then bring each case to prosecution together with the U.S. Department of Justice.
In mid-February, the FDA issued its first warning letter under the Drug Supply Chain Security Act (DSCSA) to McKesson Corp. over concerns about tampering with opioid medications. Under the DSCSA, manufacturers, repackagers, wholesale distributors, and dispensers—which are mainly pharmacies—are all required to have systems and processes in place to quarantine and investigate suspect and illegitimate medications.
This year, the agency intends publish new guidance describing its application of the risk/benefit framework for opioid analgesics in order to consider the full range of risks associated with the drugs, including risks created by illicit use. The FDA also plans to solicit input from the public to understand how it should evaluate new opioid drugs relative to those already existing on the market. New guidance will also be developed to modernize the development pathway for non-opioid analgesics to treat acute or chronic pain. As part of this push, the FDA will identify specific clinical conditions—like post-surgical pain or back pain—to speed development of new painkillers seeking narrow indications.
Also in mid-February, U.S. Sen. Patty Murray (D-WA), a ranking member of the U.S. Senate Health, Education, Labor, and Pensions (HELP) Committee, delivered highly evocative remarks at the Committee’s hearing on pain management during the opioid crisis. Murray emphasized the necessity of ensuring pain patients’ access to pain treatment while guaranteeing opioids are marketed, prescribed, and used responsibly. She also stressed that providers should be helping patients access the pain management tools best meeting their needs and called for further action to build on the steps taken last year to address the crisis.
“50 million people nationwide suffer from pain that persists for weeks or years,” Murray said in her opening remarks. “For almost 20 million people, this pain can interfere with their work and daily life activities. Pain management is an absolutely critical quality of life issue for these people and their families. Acute and chronic pain can make it harder to keep a job and earn a paycheck. Even when treated, pain can make it difficult to travel to and from work and sit at a desk for long stretches of time. And pain doesn’t just affect a person’s livelihood—it affects every aspect of their life. Without pain management, patients may not be able to tackle the tasks they need to live independently, like getting dressed, driving a car, or doing laundry. They may not be able to spend quality time with loved ones, as their pain can make it hard to enjoy a meal with friends and family, attend a grandkid’s soccer game, or even leave the house. Without the right pain management tools, some patients struggle to get a decent night’s sleep.”
Medical technologies are an integral set of tools for an effective pain management regimen that seeks to reduce or remove the number of opioid drugs involved in the treatment. These technologies range from a simple heating pad to alleviate muscle pain to targeted drug delivery devices for post-surgical recovery to a surgical or implantable neurostimulation strategy that restricts pain signals to the brain (often replacing them with paresthesia, a tingling sensation).
“Millions of Americans wake up every day suffering from chronic or acute pain, and it is critical that they have access to non-opioid treatments to help them lead productive lives,” Mark Leahey, president and CEO of the Medical Device Manufacturers Association (MDMA) commented in response to the Senate HELP Committee hearing. “Medical technologies provide life-changing relief for many who are suffering in pain, but these cures and therapies do no good if patients and physicians cannot access them. MDMA looks forward to working with Congress and all policy makers to improve access to and awareness of medical technologies so the full potential of this industry can be realized as we all work towards our common goal of ending the opioid crisis.”
As part of the efforts to address the opioid epidemic, device-delivered therapies are being considered as alternatives or adjunct to systemic opioids. One major benefit patients may find using a technology to manage pain is a greater feeling of control over their pain. Chronic pain patients not fully aware of technological alternatives may feel like they are a slave to their pills. However, the bullish strategy the White House, Congress, and the FDA are taking to mitigate this crisis may persuade patients and healthcare practitioners in a different way—patients and physicians will hopefully pursue alternative methods because the public opinion of opioid analgesics has plummeted over the past few years.
“100 million Americans have chronic pain,” explained Shai Gozani M.D., Ph.D., chairman, president, and CEO of NeuroMetrix, a Waltham, Mass.-based bioelectrical and digital medicine company driving innovation to address chronic health conditions including chronic pain and diabetes. “Many are prescribed opioids despite side effects and the potential for addiction, which has contributed to the devastating opioid crisis.
There’s a desperate need for non-pharmacological pain treatments. Recent research shows people living with chronic pain feel stigmatized by the opioid crisis and are looking for alternative treatments.1”
For far too long, opioid therapy was considered a “one-size-fits-all” solution to managing any sort of pain, acute or chronic. The drugs worked and continued to work as intended, so no alarm was raised for patients using it in a long-term setting. Managing pain is a complex task, mainly because the therapy depends on the underlying condition. Unfortunately, patients may use painkillers without understanding the complexity of their pain and the condition responsible for it. Using an opioid drug to treat a specific source of pain is like using only an air freshener to cover up a bad stench—the bad smell might be replaced with a more pleasant fragrance, but it’s going to waft right back to the nostrils unless the source of that smell is cleaned up.
Using technology in place of drugs also puts pain control in the patient’s hands. Using a drug is not something a patient actively participates in—the drug must be taken at specific intervals, and there is always the looming threat of the pain returning once the pharmaceutical has worn off. The dependence on these medicines becomes more than physical because of this mindset. Without the drug, pain patients can feel powerless to fight their condition. When using a technological solution, the patient can choose when and at what intensity to apply a therapy once their pain level becomes too high. That level of control over a previously debilitating condition can prove to be empowering for a patient who previously felt like a slave to painkillers.
“There is no one-size-fits-all approach to pain management and each person experiences pain differently,” declared Tushar. “While opioid prescriptions have long been a viable treatment for a variety of pain conditions, they have led to the drug addiction epidemic and new ways to successfully and safely treat pain are necessary for today’s patients. Innovative solutions to pain management such as device alternatives can help patients optimize their pain control to best meet their needs and improve care, while simultaneously reducing the need for opioids.”
Neurostimulation technology (neurostim for short) has been a promising avenue for non-pharmacological pain management, and a source of innovation for companies seeking to enter the newly burgeoning market. First approved by the FDA in 1989, neurostim uses modern technology and electrical pulses to manage chronic pain due to damaged or improperly functioning nerves. Neurostim devices overcome the abnormal sensations these nerves produce with a different electrical stimulus more pleasant than the original sensation—patients report feeling a tingling sensation. The technique involves implantation of a small device into the thoracic spine (for lower back or leg pain) or the neck (for upper back and arm pain). The patient essentially controls the pain with a handheld programmer.
Depending on where the electrical stimulus is applied in the body, it is either referred to as spinal cord stimulation (SCS) or peripheral nerve stimulation (PNS), both a form of neuromodulation. SCS therapy has been used to treat conditions like complex regional pain syndrome (CRPS) or failed-back surgery syndrome. According to the International Neuromodulation Society, SCS therapy now accounts for about 70 percent of all neuromodulation treatments. That number is predicted to rise to manage a variety of chronic long-term conditions.
PNS therapy is also commonly used to treat chronic pain. It involves surgery to implant a small electrical device next to one of the peripheral nerves—those located beyond the brain or spinal cord. Interestingly enough, PNS was invented in the mid-1960s, even before the commonly used SCS. Since then, it has become established for a number of clinical conditions including certain types of CRPS and pain due to peripheral nerve injuries. Some common PNS applications also include back pain treatment, occipital nerve stimulation to treat migraines, and pudendal nerve stimulation that is being investigated for urinary bladder incontinence.
“Medical technology is an important part of a pain management regimen because the technology, specific to neuromodulation, has progressed to provide effective, safe, non-addictive, reversible, and long-term solutions for chronic pain treatment,” said Mark Geiger, global director of marketing, implantables, for Bioness Inc., a Valencia, Calif.-based medical device company that provides technologies helping people to regain mobility and independence. The company produces implantable and external neuromodulation systems, robotic systems, and software-based therapy programs providing functional and therapeutic benefits for those affected by pain, central nervous system disorders, and orthopedic injuries. “Many neuromodulation devices, including PNS products like the StimRouter, are minimally invasive and only require a 15-30-minute implant by a doctor while the patient is awake under local anesthesia. These devices can put the patient in charge of their own pain management at home.”
Bioness’ StimRouter first entered the U.S. market in 2015 following FDA clearance. StimRouter uses PNS therapy to treat intractable pain originating in a peripheral nerve. Applications for the technology include chronic pain conditions located at or related to the upper and lower limbs, entrapment syndromes, intercostal neuralgias, or other peripheral injuries or diseases. The StimRouter implant consists of a passive, 15 cm lead with electrodes at one end and a receiver at the other that is implanted percutaneously with a small incision under local anesthesia. The lead’s tip is placed near the peripheral nerve causing the chronic pain.

StimRouter is the only implantable neuromodulation device currently marketed for adults with chronic pain of peripheral nerve origin. Image courtesy of Bioness.
An External Pulse Transmitter “patch” is worn on the skin over the implant, powering it and sending the stimulation signal to the implant. The patient controls their desired stimulation with a “Patient Programmer” resembling a small remote control. The programmer turns the system on, off, up, and down to control the pain, and is equipped with a display to monitor and manage stimulation programs and the level of stimulation intensity.
“The StimRouter was the first system of its kind designed specifically to treat focal, chronic pain of a peripheral nerve origin,” said Geiger. “When compared to the more established spinal cord stimulation systems offered by several large medical device corporations (which involves major surgery with a large, global implant), the StimRouter has the electronics (processor, battery) residing on the wearable outside the body, resulting in a very small, simple implant process while the patient is awake. This eliminates many of the risks typically associated with a spinal cord stimulator, such as implant migration.”
According to the Rheumatoid Arthritis Support Network, as of 2018 rheumatoid arthritis (RA) affected over 1.3 million Americans and as much as 1 percent of the entire world population. The autoimmune disease mainly attacks the synovial tissues within the joints. RA can affect the entire body, and can attack organs like the heart, lungs, or tissues like muscles, cartilage, and ligaments. RA causes chronic swelling and pain that can be severe, and can cause permanent disability.
The pain experienced by RA patients manifests differently than neuropathic pain, requiring different treatment options as a result. Typically, biologic therapies are used to address the underlying chronic inflammation, which may reduce patients’ pain. Unfortunately, biologic therapies can suppress the immune system and aren’t always effective, leading to refractory disease. There is an enormous unmet need for new treatments that are more effective, less immunosuppressive, and more cost-effective than current therapies as a result.
“Despite significant progress with pharmacological therapy, 30-50 percent of RA patients either don’t adequately respond to or are intolerant of biologic therapies,” explained Murthy Simhambhatla, Ph.D., CEO of SetPoint Medical, a Valencia, Calif.-based bioelectronic medicine company dedicated to treating patients with chronic autoimmune diseases. “These therapies can have serious side effects and impose a significant cost burden on the healthcare system. In fact, some of the highest revenue-grossing drugs in history are indicated for patients with RA and other inflammatory autoimmune diseases, and these drugs come with FDA black box warnings regarding serious side effects. Furthermore, patient compliance is a major issue as these drugs typically are injectable therapies.”
Bioelectronic medicine (i.e., nerve stimulating or nerve blocking technology) is a fairly new approach to treat disease and injury. When implanted on a nerve or held against the skin, bioelectronic medical devices can potentially modulate specific nerve activity, elicit a specific change in organ function, and restore health without the complicated side effects of pharmaceuticals.
“We are pioneering the use of bioelectric medicine to fundamentally alter the treatment paradigm for autoimmune diseases,” said Simhambhatla. “Bioelectronic medicine activates a natural physiologic pathway through vagus nerve stimulation to restore immunological homeostasis—or, return the body to its natural ‘set point.’”
The company’s bioelectronic medicine platform delivers targeted electronic pulses to trigger the intrinsic inflammatory reflex. That pathway has found promise as a treatment option for RA, Crohn’s disease, and other chronic autoimmune diseases. SetPoint’s bioelectronic platform consists of a small implantable MicroRegulator, a wireless charging collar or band, and an iPad-based prescription application.

SetPoint Medical's miniature bioelectronic medicine device delivers electrical doses designed to activate the innate inflammatory reflex to produce a systemic anti-inflammatory effect and help restore balance and regulate the immune system. Image courtesy of SetPoint Medical.
“SetPoint’s miniature (less than one inch long) bioelectronic medicine device is implanted on the left vagus nerve via a small incision in the neck. It delivers electrical impulses designed to activate the innate inflammatory reflex to produce a systemic anti-inflammatory effect, and to help restore balance and regulate the immune system.”
The company recently completed its U.S. Pilot IDE Study in patients with multi-drug refractory RA. The study was successful in further validating the device’s mechanism, indicating that it was safe and well-tolerated. According to Simhambhatla, SetPoint anticipates initiating a larger clinical study to evaluate the bioelectronic platform’s safety and efficacy in RA patients in the second half of this year.
Transcutaneous electrical nerve stimulation (TENS) therapy use low-voltage electric currents to treat pain. Electrodes or mediums for electricity to travel to the body are placed on the site of pain, and the currents block pain receptors from being sent from the nerves to the brain. Usually the patient will receive a small, battery-operated TENS machine to use at home. TENS therapy can be used to treat both chronic and acute pain, and TENS is most commonly used to treat osteoporosis- or fibromyalgia-related joint, bone, or muscle problems, tendinitis, bursitis, and neck, labor, and cancer pain.
NeuroMetrix’s Quell, a wearable, over-the-counter (OTC) TENS device, was announced at the 2015 Consumer Electronics Show (CES). The device is worn on the leg and sends neural pulses to the brain that trigger a natural pain relief response in the central nervous system. According to the company, since first launching Quell, almost 200,000 individuals living with chronic pain have experienced its patented neurotechnology.

Wearable technology, through miniaturization, automation, and user-centric design allows a person living with chronic pain to receive treatment and experience relief whenever they need it. Image courtesy of NeuroMetrix.
Quell 2.0, the next-generation iteration of the wearable TENS device, was launched at this year’s CES. Quell 2.0 is about 50 percent smaller than the original product, but features a larger battery to provide more therapy sessions per charge. It is powered by the Quell Relief App, which lets users calibrate the device, start and stop therapy, adjust intensity, and track sleep, activity, pain, and gait. It enables customized therapy dosages, sleep modes, and can adapt to changes in local weather, providing user alerts and the option to increase dosage if the weather is predicted to change in a manner that affects the user’s pain.
“Quell is a more advanced way to treat chronic pain,” said Dr. Gozani. “Like TENS units and implantable spinal stimulators, Quell uses electrical nerve stimulation to provide pain relief. Quell 2.0 is up to 10x more powerful than typical OTC TENS devices.2 Quell is also the only OTC electrical nerve stimulation device FDA cleared for use while sleeping and can be used during the day when active or at night.”

Chronic pain affects many people 24/7, making it important for a technolgy solution to fit seamlessly into their life. Image courtesy of NeuroMetrix.
The company is looking to bring even more personalization to Quell in the future, and expects to upgrade Quell 2.0’s firmware later this year. The upgrade will allow the technology to deliver on the promise of artificial intelligence. The algorithms will be derived from the application of machine learning to millions of data points from over 70,000 chronic pain sufferers in the company’s Quell Health Cloud.
“The process evaluates demographics, health conditions, pain levels and characteristics, device utilization, and objective measures of sleep, activity, and gait from Quell users to power truly personalized treatment,” explained Dr. Gozani. “Quell is already smart enough to adjust a user’s therapy based on manual intensity changes, body position, and sleep movements. With this upgrade, setup and use will be even more precise.”
Avanos Medical’s ON-Q* Pain Relief System aims to reduce the number of opioids used for post-surgical recovery. The opioid-sparing elastomeric ambulatory infusion pump automatically and continuously delivers a regulated flow of local anesthetic to a surgical site or in close proximity to nerves, providing targeted pain relief for up to five days. It also features a variable rate controller that changes flow rate according to patients’ individual pain relief requirements.
“Patients treated with ON-Q* may experience faster recovery and less post-operative pain with fewer side effects than patients taking opioids alone,” said Tushar. “ON-Q* may help surgical patients to avoid taking that first opioid, which could otherwise lead to an addiction.”
Avanos’ COOLIEF* Cooled Radiofrequency (RF) is a thermal RF system that uses water-cooled technology (hence the name) to deactivate pain-causing sensory nerves. It works via a RF generator that transmits a small current of energy through an insulated electrode placed within tissue. The friction of charged molecules produces ionic heating, which thermally deactivates the offending nerves. According to the company, delivering RF energy through water-cooled electrodes allows more energy to be safely delivered to target nerves and create ideal spherically-shaped lesions. The system can currently be used to treat chronic sacroiliac joint pain, symptomatic discogenic pain, lumbar medial branch neurotomy, chronic thoracic facet pain, and cervical pain.
Last year, the company acquired Game Ready, a cold and intermittent compression therapy system used for injury and recovery from orthopedic surgery. The system delivers targeted cold and compression therapy through anatomically-designed wraps to help reduce pain and swelling, and reduce opioid consumption.
“Adding this market-leading technology to our portfolio of non-opioid treatment options further commits the company to the innovative fight to reduce the use of opioids,” declared Tushar.
Fight on.
References
- NeuroMetrix. (2018). Flipping the Script: Living with Chronic Pain amid the Opioid Epidemic.
- Comparison to leading OTC TENS devices, based on IRI data. Max sustained energy when used as directed.
More information about opioid-sparing pain management and the technologies featured in this article is available via the interviews below: